|
|
|
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.
Excessive alcohol use costs the country approximately $235 billion every year.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.
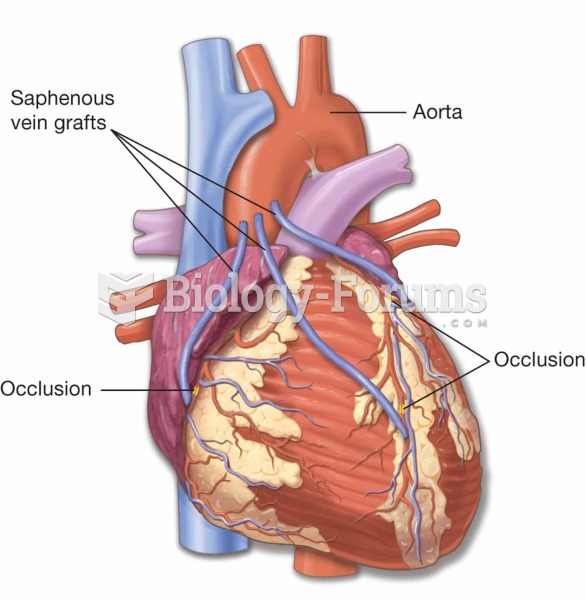 A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
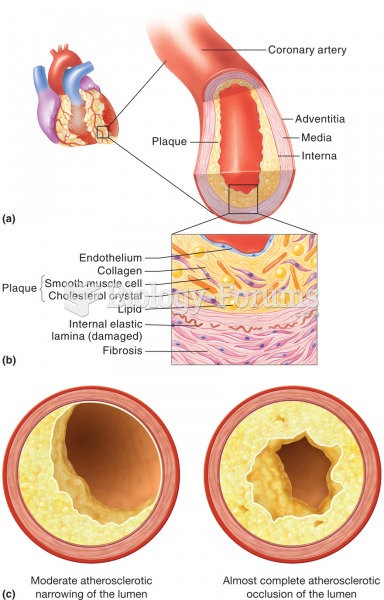 Atherosclerosis. (a) A sectioned coronary artery that exhibits an accumulation of fatty plaque, whic
Atherosclerosis. (a) A sectioned coronary artery that exhibits an accumulation of fatty plaque, whic





