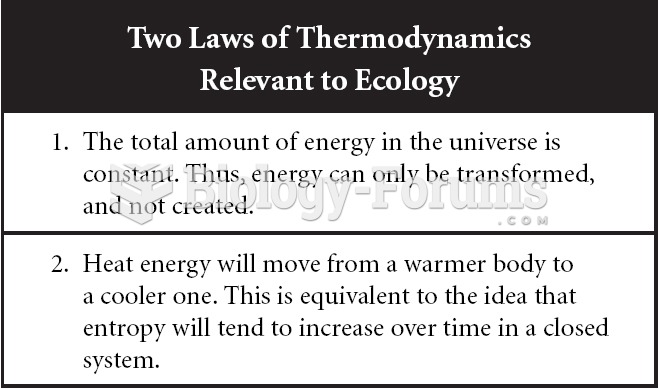
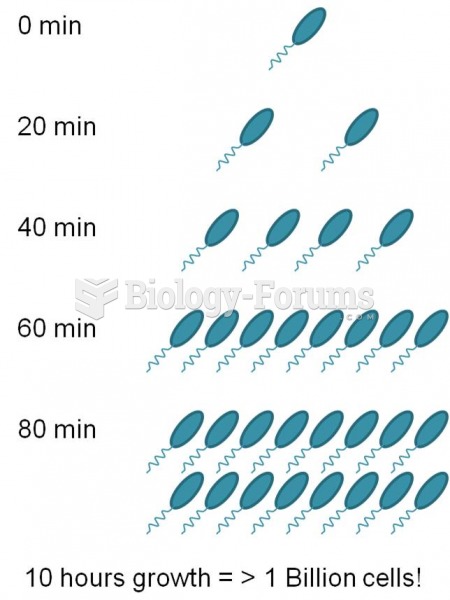
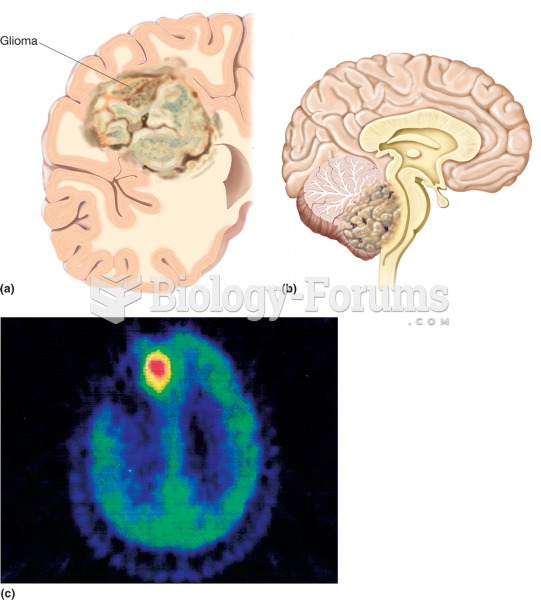
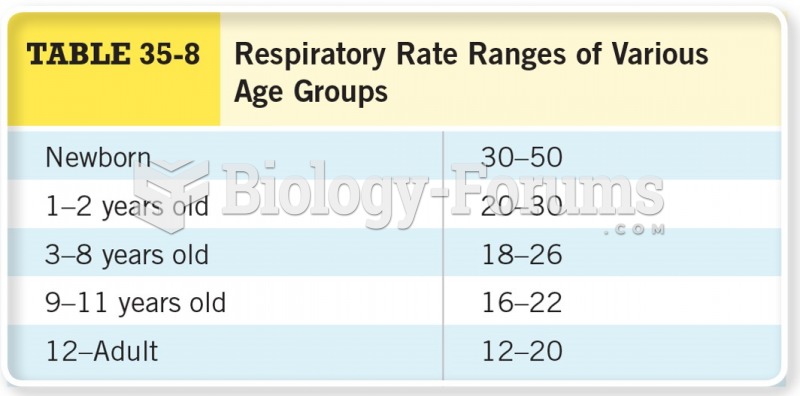
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.