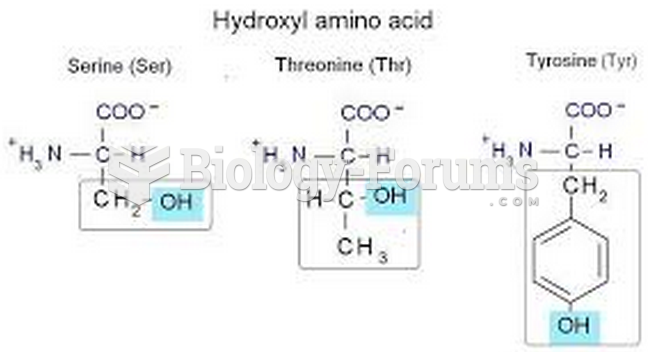
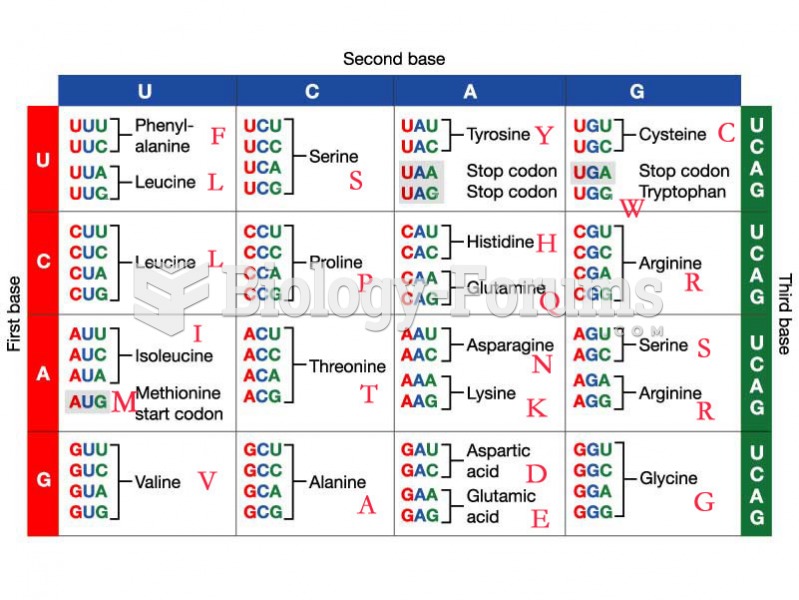
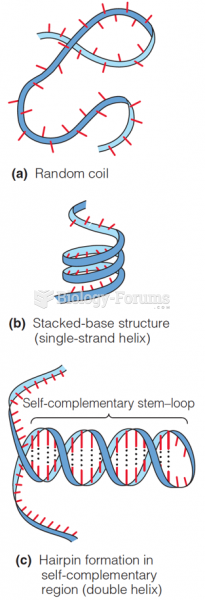
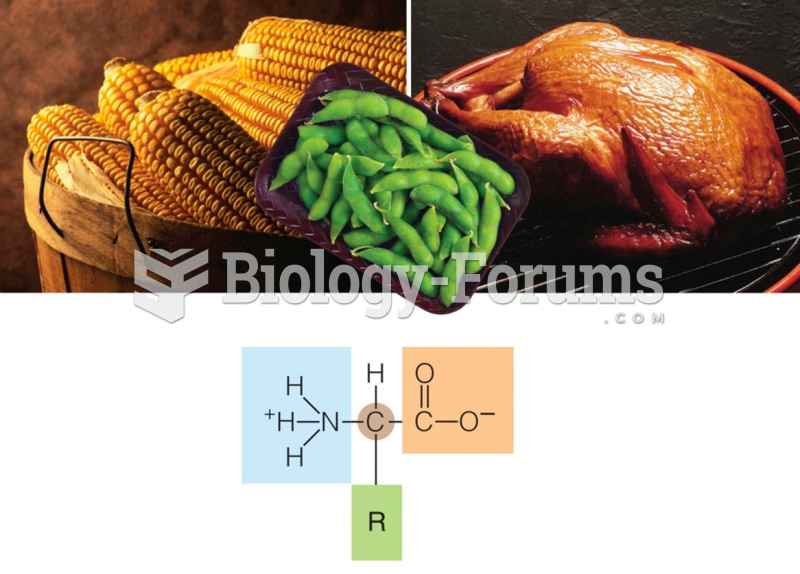
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.