|
|
|
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
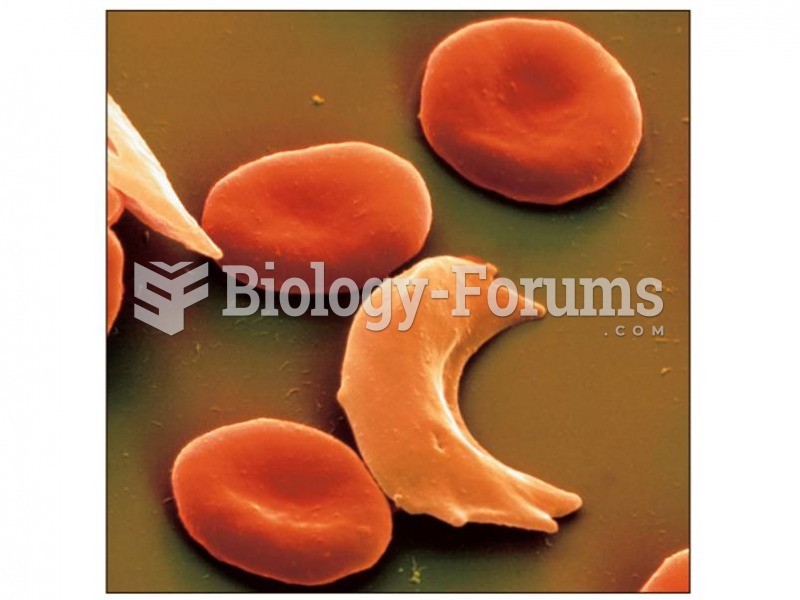
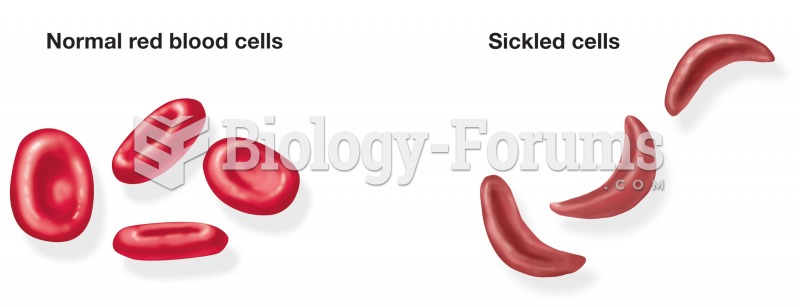 Comparison of normal-shaped erythro- cytes and the abnormal sickle shape noted in patients with sick
Comparison of normal-shaped erythro- cytes and the abnormal sickle shape noted in patients with sick
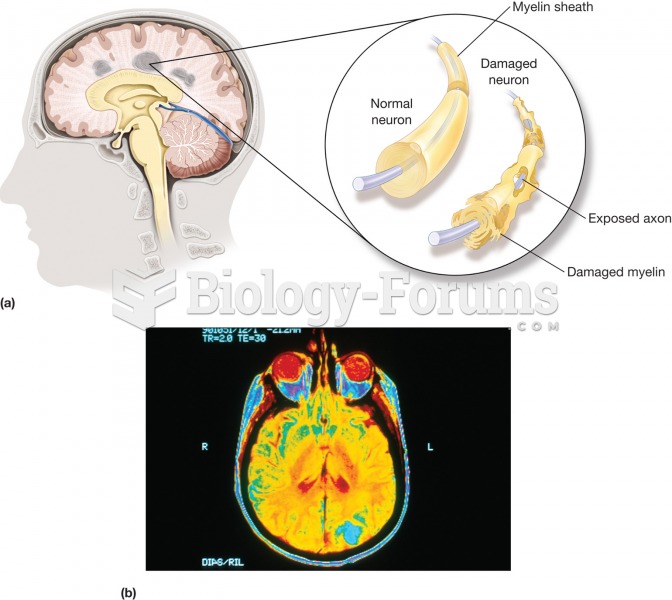 Multiple sclerosis (MS). (a) A disease characterized by the gradual development of small areas of ha
Multiple sclerosis (MS). (a) A disease characterized by the gradual development of small areas of ha