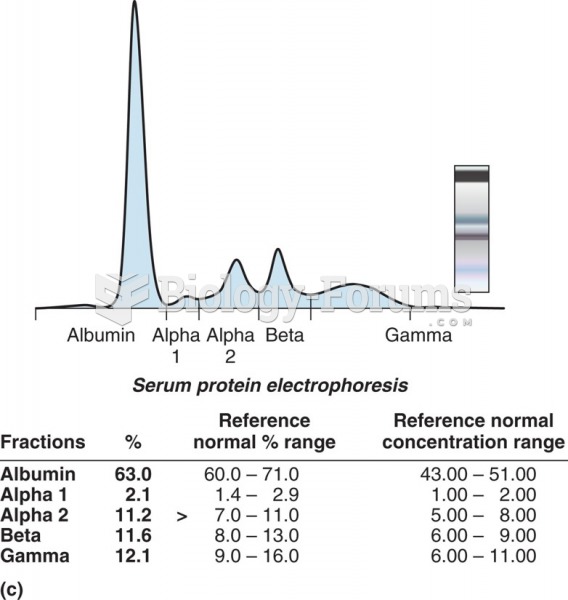
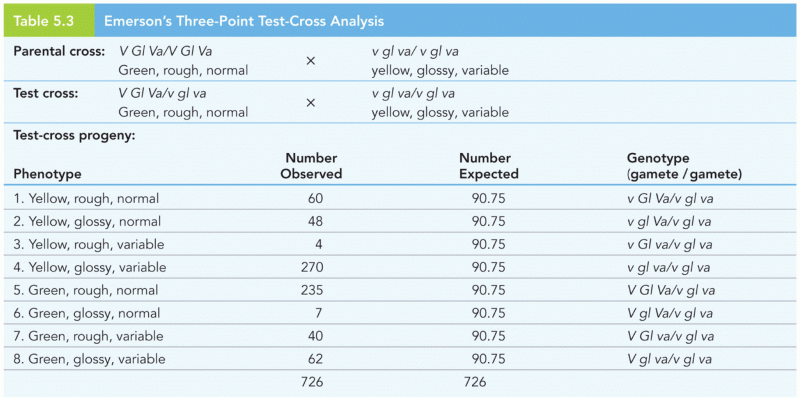
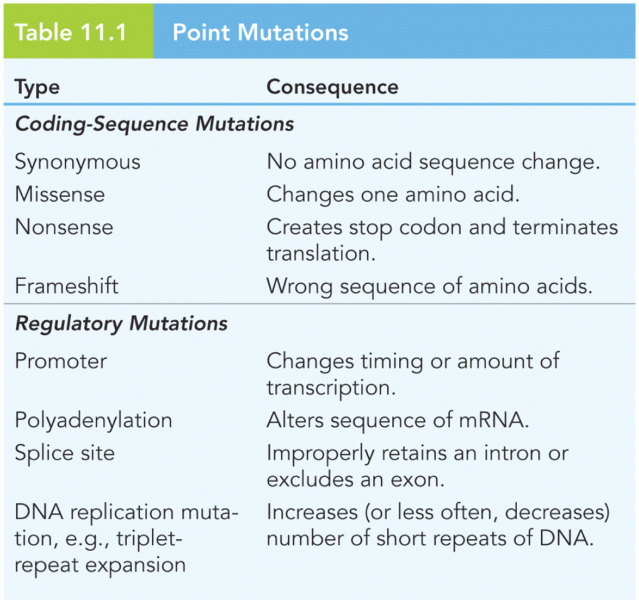
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
Pink eye is a term that refers to conjunctivitis, which is inflammation of the thin, clear membrane (conjunctiva) over the white part of the eye (sclera). It may be triggered by a virus, bacteria, or foreign body in the eye. Antibiotic eye drops alleviate bacterial conjunctivitis, and antihistamine allergy pills or eye drops help control allergic conjunctivitis symptoms.