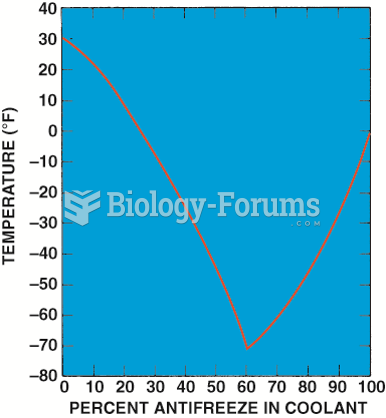
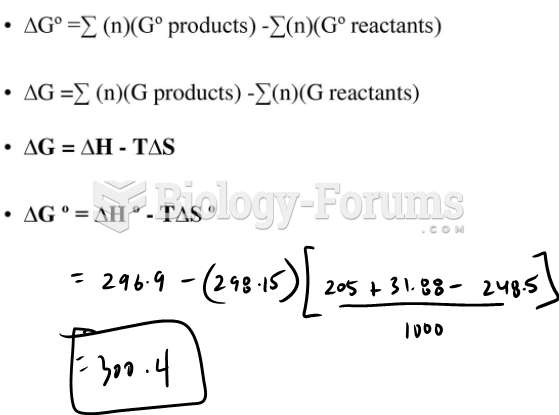
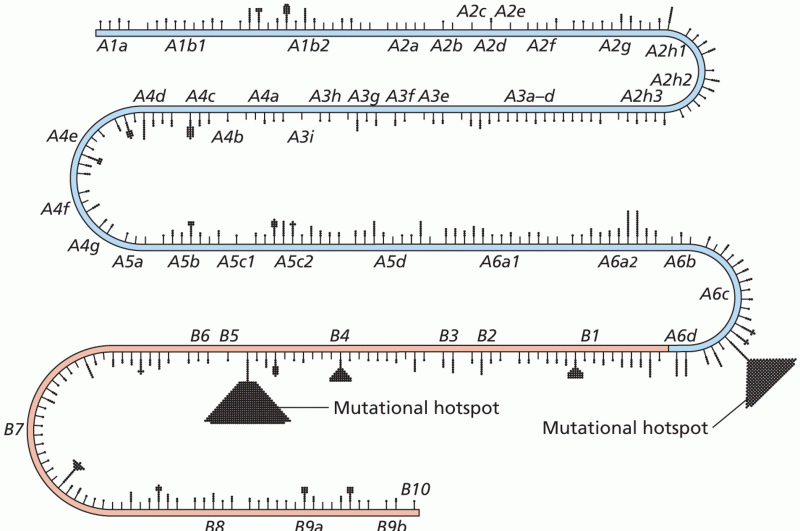
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.