|
|
|
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
A cataract is a clouding of the eyes' natural lens. As we age, some clouding of the lens may occur. The first sign of a cataract is usually blurry vision. Although glasses and other visual aids may at first help a person with cataracts, surgery may become inevitable. Cataract surgery is very successful in restoring vision, and it is the most frequently performed surgery in the United States.
More than 4.4billion prescriptions were dispensed within the United States in 2016.
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
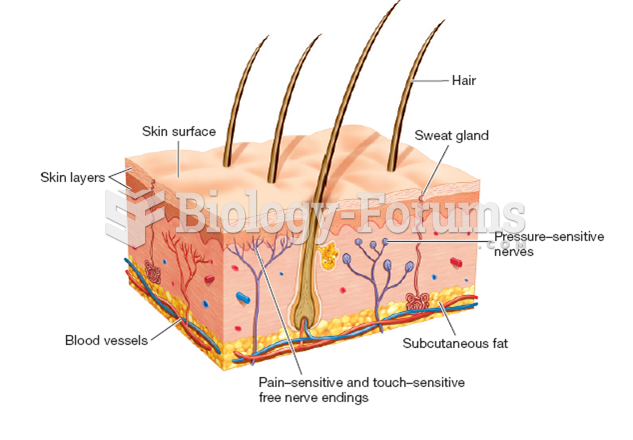
If you could remove all of your skin, it would weigh up to 5 pounds.
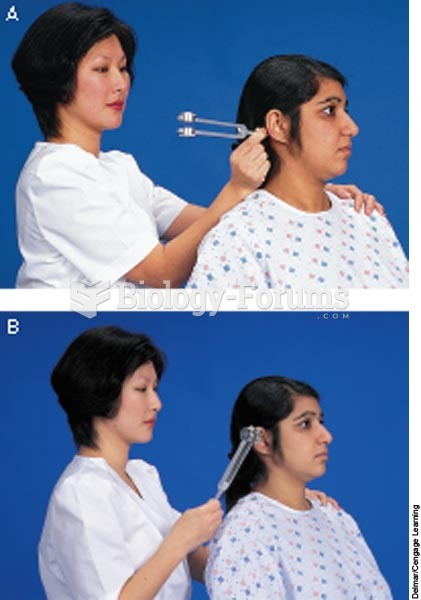 Rinne test: A. Place the base of the tuning fork on the mastoid process. B. Place tuning fork in fro
Rinne test: A. Place the base of the tuning fork on the mastoid process. B. Place tuning fork in fro
 A Corner of the General Massage Section of an Army Hospital. A Practice of Physiotherapy by Sampson, ...
A Corner of the General Massage Section of an Army Hospital. A Practice of Physiotherapy by Sampson, ...