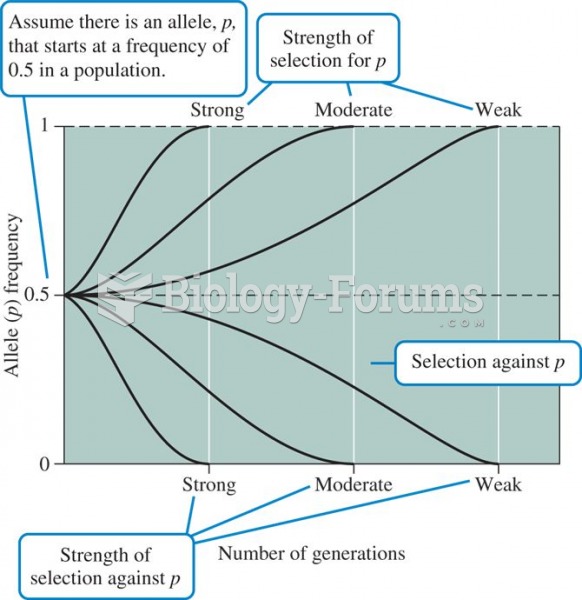
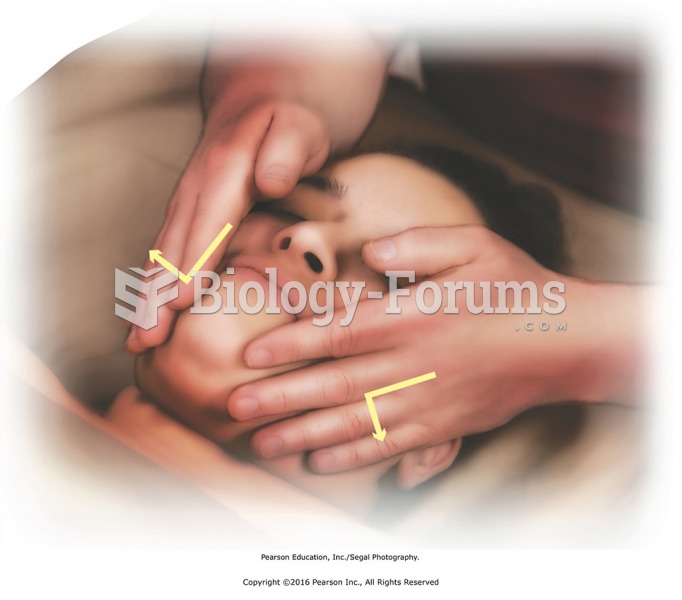
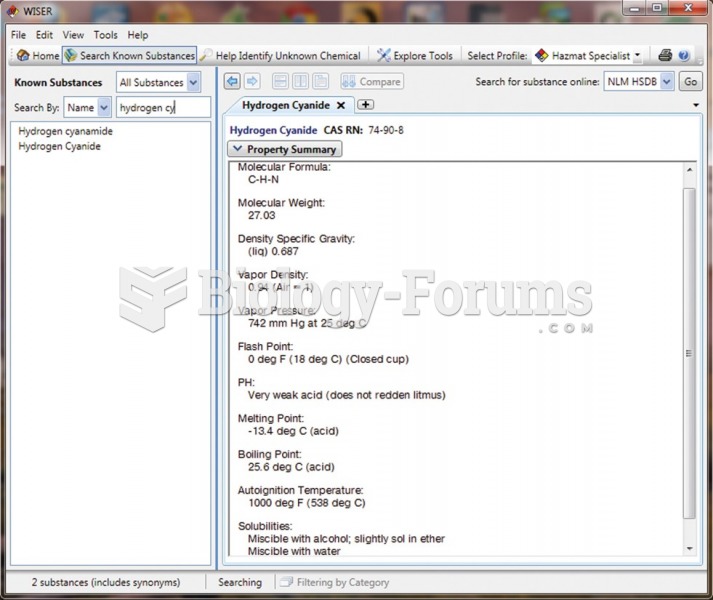
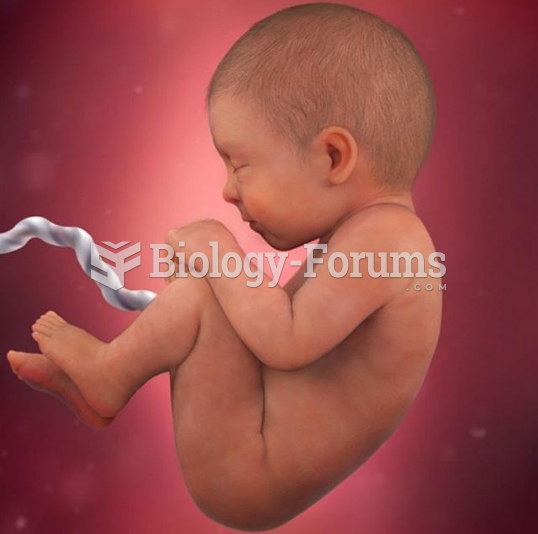
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.