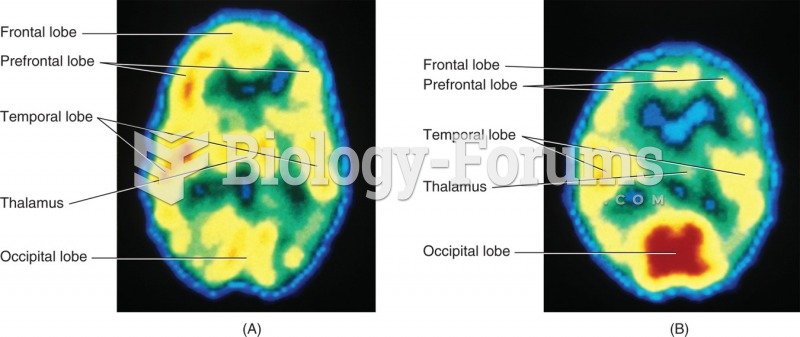
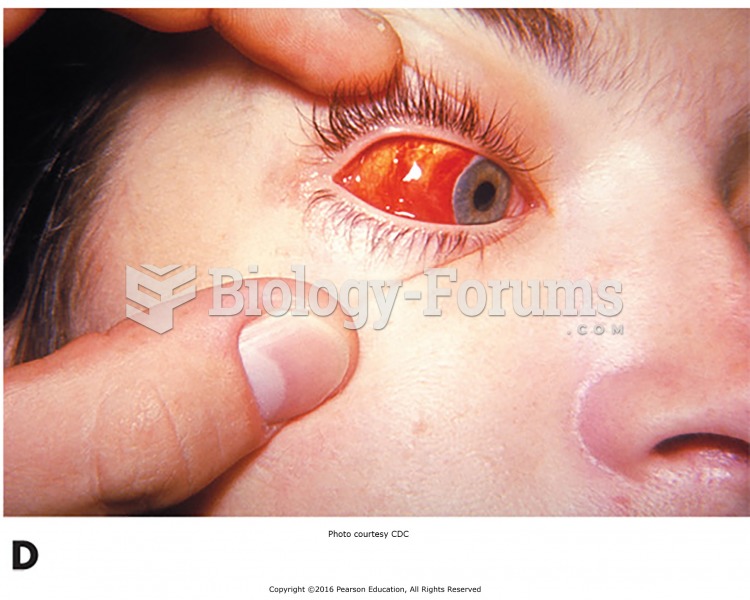
|
|
|
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Before a vaccine is licensed in the USA, the Food and Drug Administration (FDA) reviews it for safety and effectiveness. The CDC then reviews all studies again, as well as the American Academy of Pediatrics and the American Academy of Family Physicians. Every lot of vaccine is tested before administration to the public, and the FDA regularly inspects vaccine manufacturers' facilities.
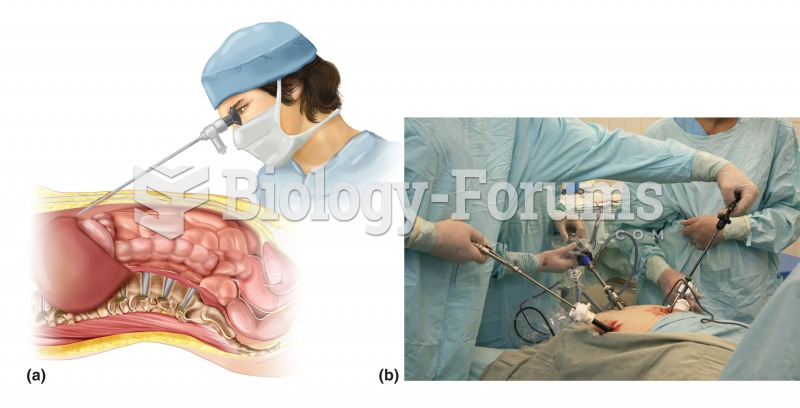 GI endoscopy. (a) Similar to other forms of GI endoscopy, laparoscopy involves the insertion of a sp
GI endoscopy. (a) Similar to other forms of GI endoscopy, laparoscopy involves the insertion of a sp
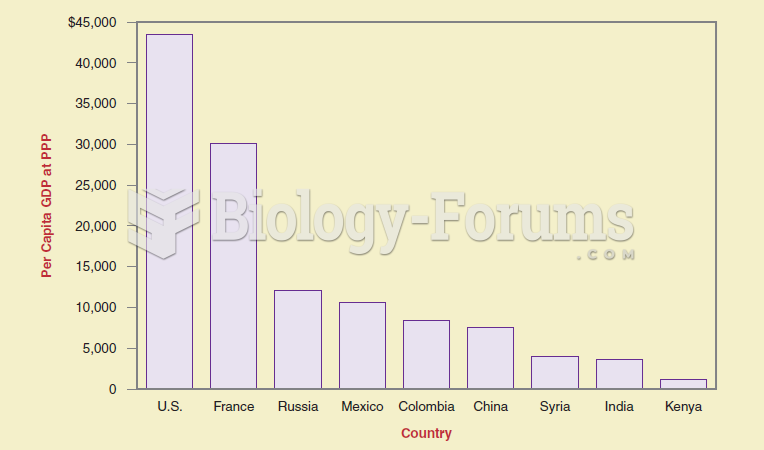 If you want to show differences between items at the same time, a bar graph is more effective than a
If you want to show differences between items at the same time, a bar graph is more effective than a
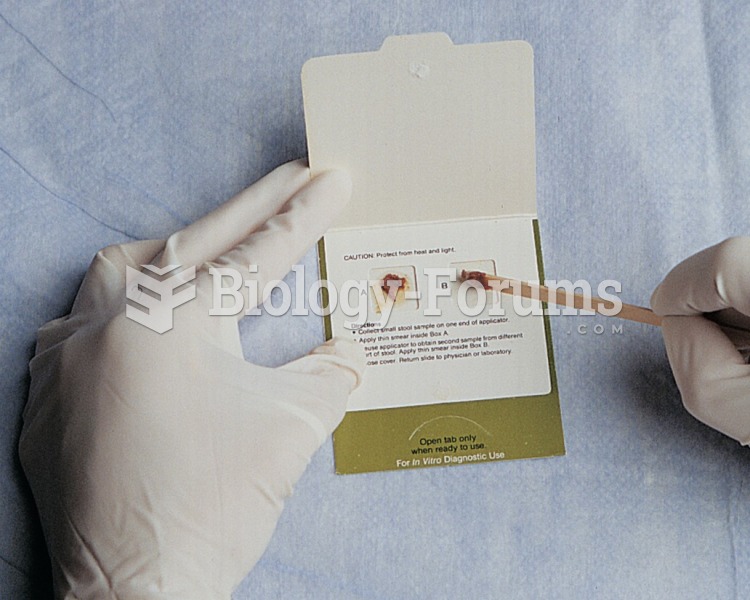 Instruct the patient to use an applicator to apply a small amount of stool in the appropriate boxes ...
Instruct the patient to use an applicator to apply a small amount of stool in the appropriate boxes ...