|
|
|
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
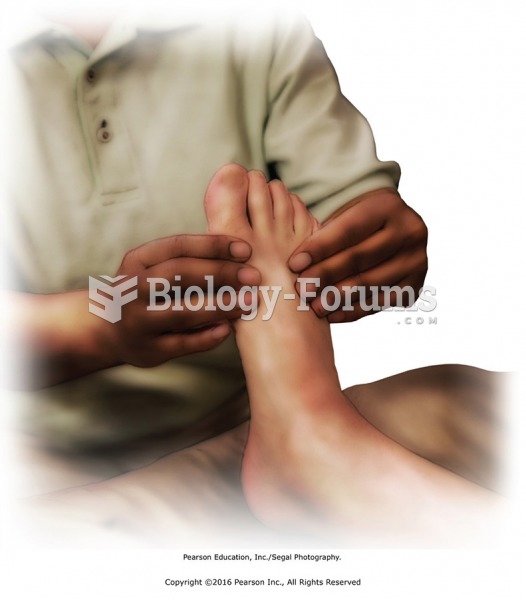
Children with strabismus (crossed eyes) can be treated. They are not able to outgrow this condition on their own, but with help, it can be more easily corrected at a younger age. It is important for infants to have eye examinations as early as possible in their development and then another at age 2 years.
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
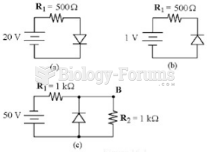
Disorders that may affect pharmacodynamics include genetic mutations, malnutrition, thyrotoxicosis, myasthenia gravis, Parkinson's disease, and certain forms of insulin-resistant diabetes mellitus.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
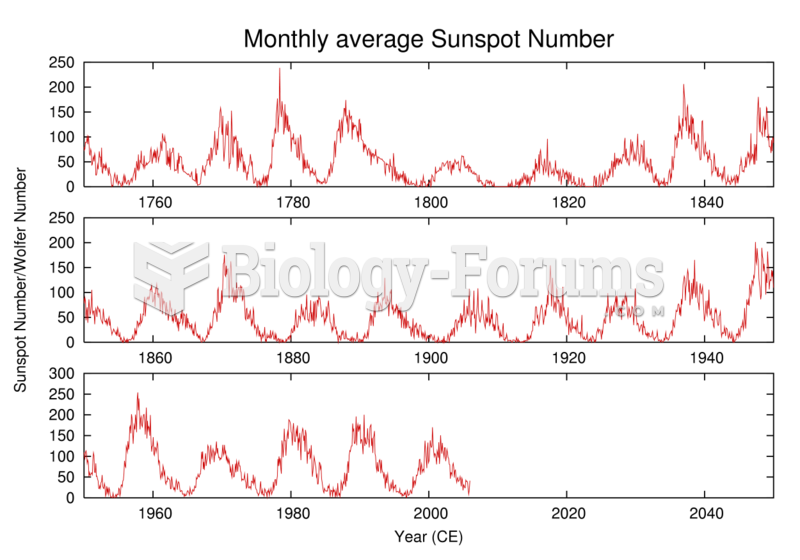 History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar
History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar
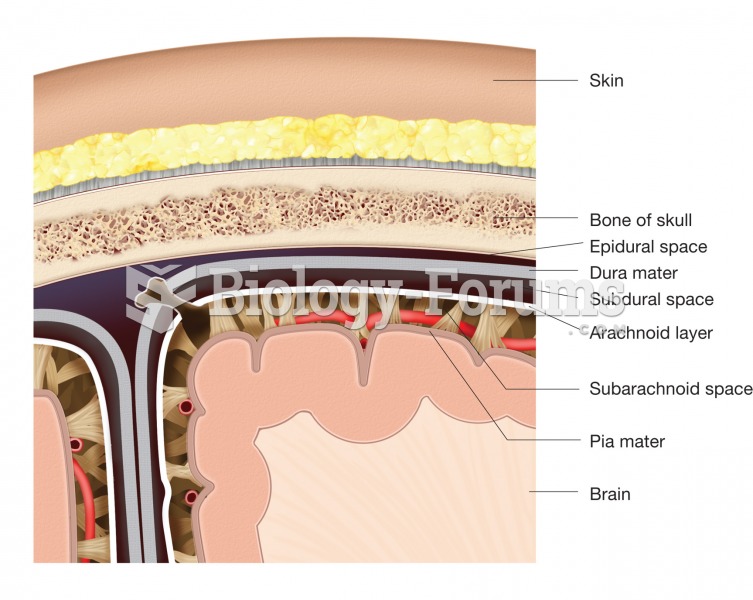 The meninges. This figure illustrates the location and structure of each layer of the meninges and t
The meninges. This figure illustrates the location and structure of each layer of the meninges and t