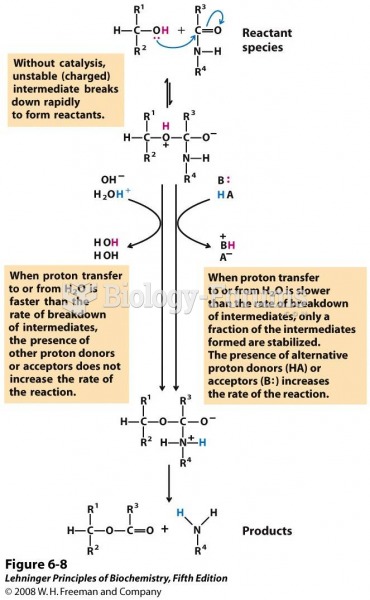
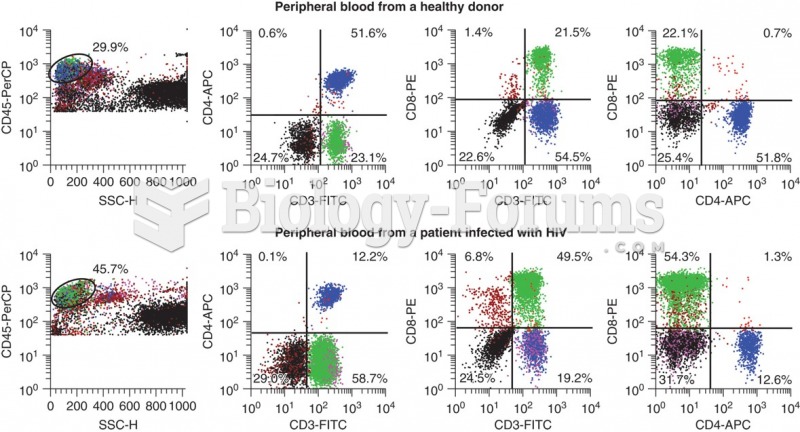
|
|
|
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
 Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
 In this cartoon, Boss Tweed welcomes cholera—a skeletal figure of death carrying a handbag from “Asi
In this cartoon, Boss Tweed welcomes cholera—a skeletal figure of death carrying a handbag from “Asi