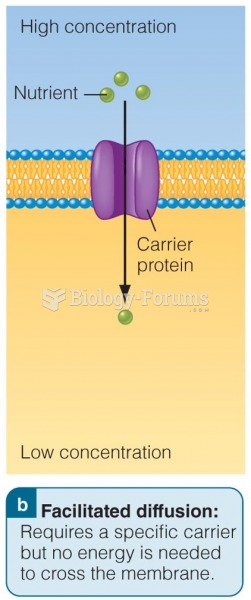
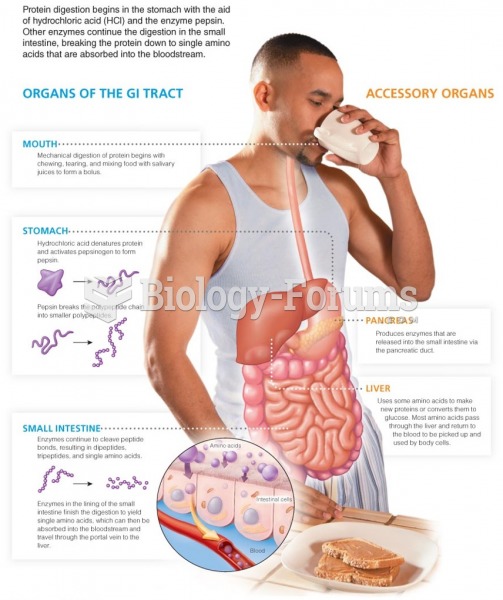
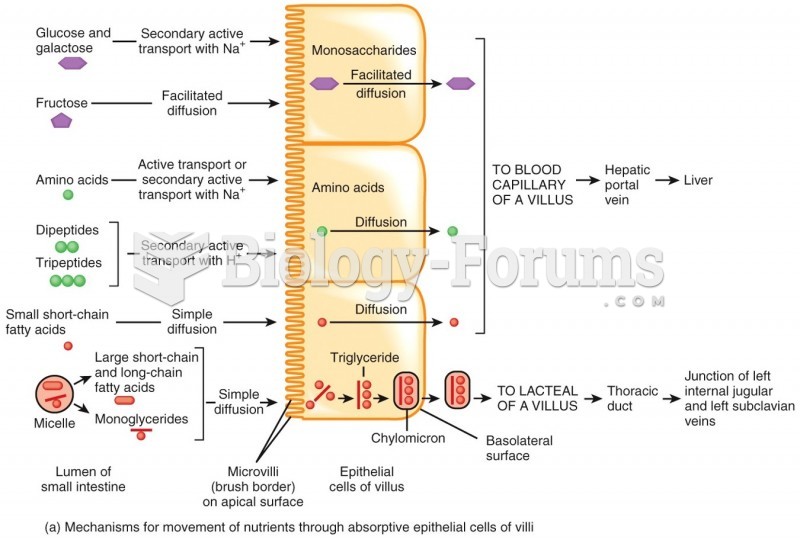
|
|
|
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
The horizontal fraction bar was introduced by the Arabs.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.