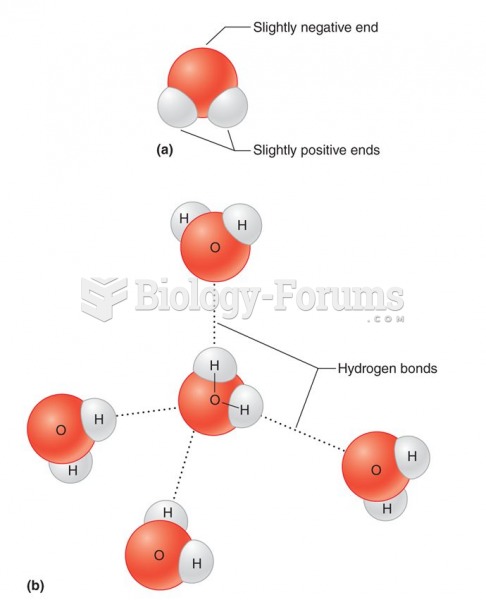
Answer to Question 1Because of the way a water molecules oxygen electrons are distributed, the overall geometry
of the molecule is a bent or angular shape. The angle formed by the two hydrogen atoms and
the central oxy-gen atom is about 105. The angular shape of the water molecule makes it
electrically asymmetrical. Each water molecule can be thought of as having a positive (1) end
and a negative (2) end. This is because protons of the hydrogen atomsthe positively
charged particles in the nucleus (center)are left partially exposed when the negatively
charged electrons bond more closely to oxygen. The water molecule behaves something like
a magnet: its positive end attracts particles that have a negative charge, and its negative end
(or pole) attracts particles that have a positive charge. For this reason, water is called a polar
molecule. When water comes into contact with compounds whose elements are held together
by the attraction of opposite electrical charges (most salts, for example), the polar water
molecule will separate that compounds component elements from each other. This explains
why water can easily dissolve so many other compounds. The polar nature of water also
permits it to attract other water molecules. When a hydrogen atom (the positive end) in one
water molecule is attracted to the oxygen atom (the negative end) of an adjacent water
molecule, a hydrogen bond forms. A hydrogen bond between molecules is about 5 to 10
as strong as a covalent bond within a molecule. Hydrogen bonds link water molecules
together by electrostatic forces. Hydrogen bonds greatly influence the properties of water by
allowing individual water molecules to stick to each other, a property called cohesion.
Cohesion gives water an unusually high surface tension. Adhesion, the tendency of water to
stick to other materials, enables water to adhere to solidsthat is, to make them wet.
Cohesion and adhesion are the causes of capillary action, the tendency of water to spread
through a towel when one corner is dipped in water.
Answer to Question 2Liquid waters thermal characteristics prevent broad swings of temperature during day and
night, and, through a longer span, during winter and summer. Heat is stored in the ocean
during the day and released at night. A much greater amount of heat is stored through the
summer and given off during the winter. Liquid water has an important thermostatic
balancing effectan oceanless Earth would be much colder in winter and much hotter in
summer than the moderate temperatures we experience.