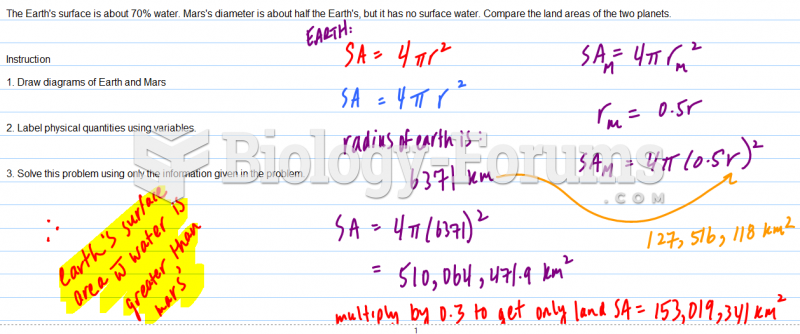
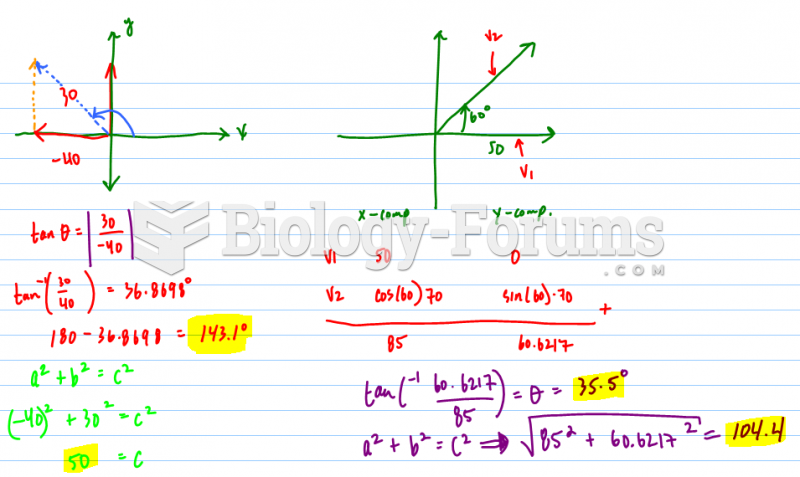
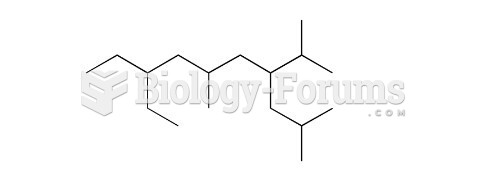
|
|
|
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.