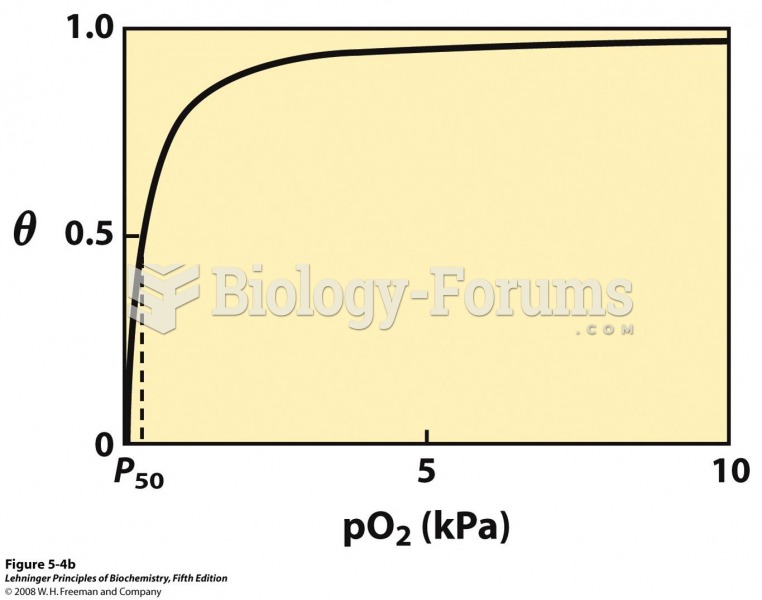
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Most strokes are caused when blood clots move to a blood vessel in the brain and block blood flow to that area. Thrombolytic therapy can be used to dissolve the clot quickly. If given within 3 hours of the first stroke symptoms, this therapy can help limit stroke damage and disability.