|
|
|
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
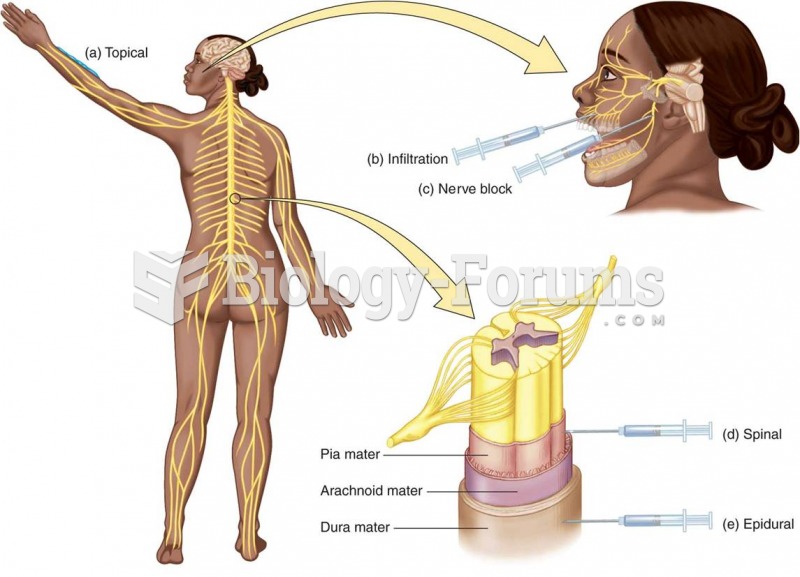 Techniques for applying local anesthesia: (a) topical; (b) infiltration; (c) nerve block; (d) spinal
Techniques for applying local anesthesia: (a) topical; (b) infiltration; (c) nerve block; (d) spinal
 The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p
The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p