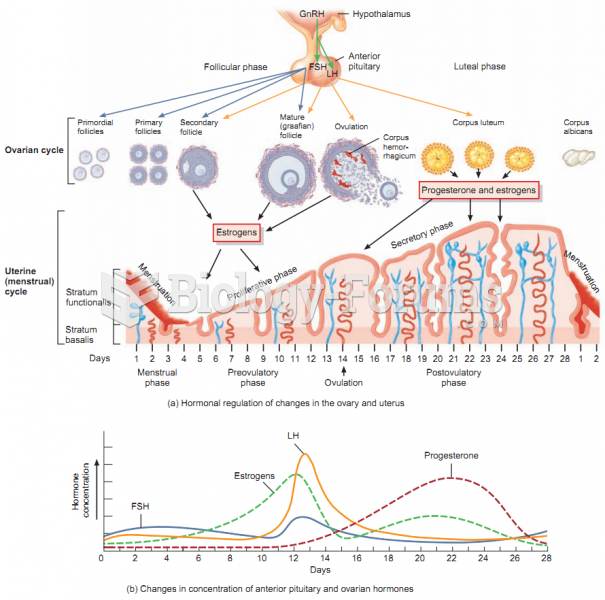
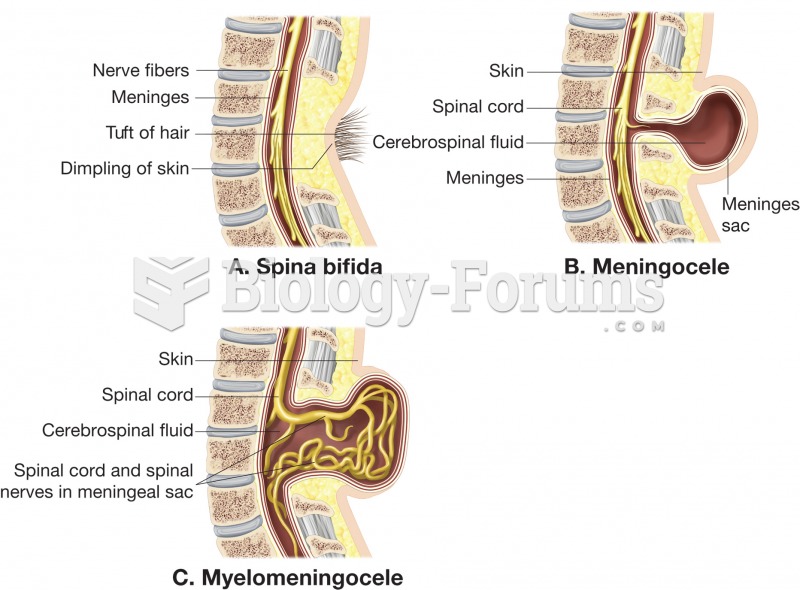
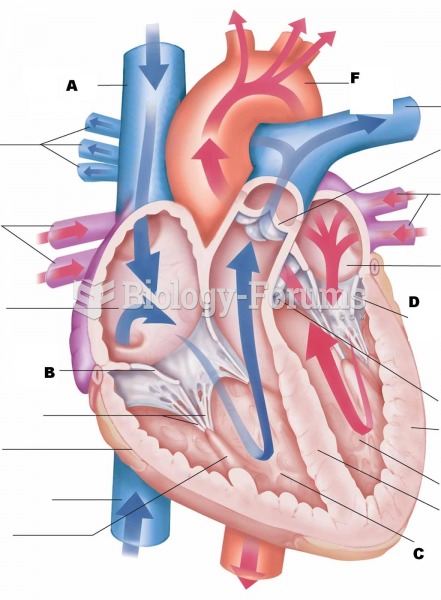
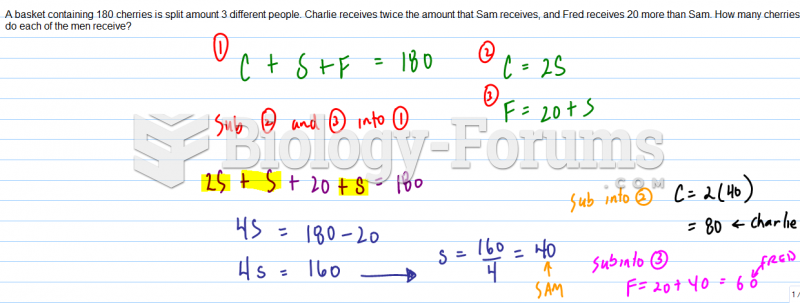
Consider the cycloheptatrienyl anion. Complete the following questions by filling in the blank with the appropriate word.
According the Hckel criteria this anion would be classified as ______________.
Question 2Answer the following questions concerning sulfathiazole below by filling ieach blank with the appropriate response.

The hybridization of the nitrogen atom in sulfathiazole is ______.
A. sp
B. sp2
C. sp3
Question 3Consider the following general structure.

Positions 1,4 and 2,6 and 3,5 are termed
para,
ortho, and
meta, respectively.
a.
True
b.
False
Question 4The following compound could be produced by reaction of phenol with bromobenzene.

a.
True
b.
False
Question 5Consider the following structure.

The
1H NMR spectrum of this compound 60C shows a peak at 7.6 ppm, this would indicate aromaticity.
a.
True
b.
False
Question 6The following compound should be name as 1-((1
S,
3R)-3-methylcyclohexyl)benzene.

a.
True
b.
False
Question 7The product of the following reaction would be 1-bromo-1-phenylethane.

a.
True
b.
False
Question 8The product of the following reaction would be a dicarboxylic acid.

a.
True
b.
False
Question 9Consider the following structures,

A B
Structure A is more acidic than B.
a.
True
b.
False
Question 10Furan has the structure shown below.

The orbitals in furan are shown below.

a.
True
b.
False
Question 11The following compound is named (
Z)-2,3-diphenyl-2-hexene.

a.
True
b.
False
Question 12Which of the following does not undergo oxidation in the presence of H2CrO4?

a.
1
b.
2
c.
3
d.
4
Question 13Which of the following is not true about the bridged 10annulene shown below?

a.
all of the carbon atoms of bridged 10annulene are all in the same plane
b.
bridged 10annulene is aromatic
c.
bridged 10annulene undergoes substitution reactions similar to benzene
d.
bridged 10annulene has 10 pi electrons
Question 14Which of the following is true about 10annulene?

a.
10annulene is planar
b.
10annulene is nonaromatic
c.
10annulene undergoes addition reactions similar to simple alkenes
d.
10annulene has 10 pi electrons
Question 15Which of the following is not true about 18annulene?

a.
18annulene is planar
b.
18annulene is aromatic
c.
18annulene gives one peak in the
1H NMR spectrum
d.
18annulene has 18 pi bonds
Question 16What are the relative positions of the substituents in the following structure?

a.
anti
b.
meta
c.
ortho
d.
para
Question 17What are the relative positions of the substituents in the following structure?

a.
anti
b.
meta
c.
ortho
d.
para
Question 18What are the relative positions of the substituents in the following structure?

a.
anti
b.
meta
c.
ortho
d.
para
Question 19Which of the following represents the energy levels of the molecular orbitals of cyclobutadiene?