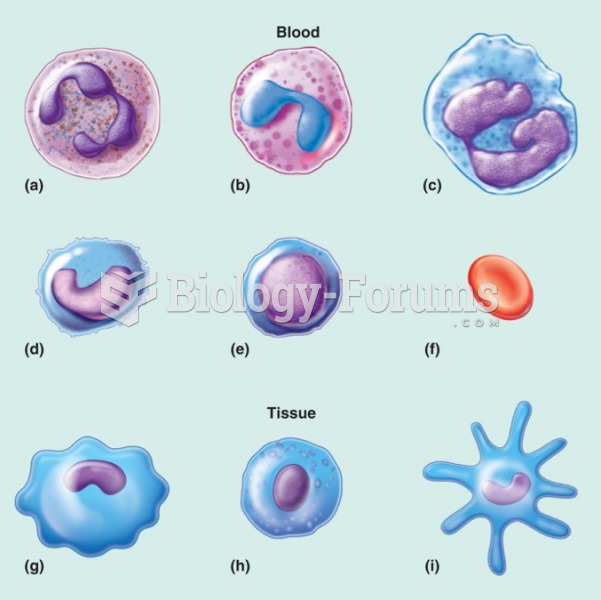
|
|
|
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
The average adult has about 21 square feet of skin.
 These two species of bedstraw grow predominately on different soil types: Galium saxatile (shown her
These two species of bedstraw grow predominately on different soil types: Galium saxatile (shown her
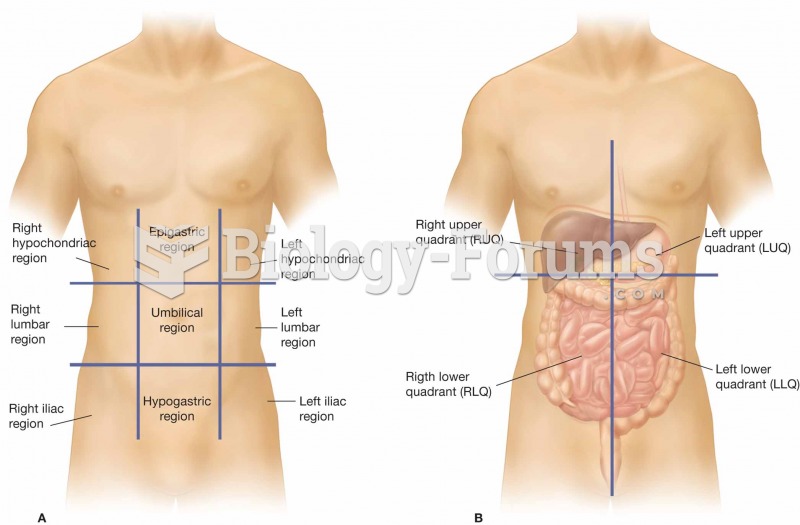 (A) The nine regions of the abdominopelvic cavity. (B) The four regions of the abdomen, which are re
(A) The nine regions of the abdominopelvic cavity. (B) The four regions of the abdomen, which are re
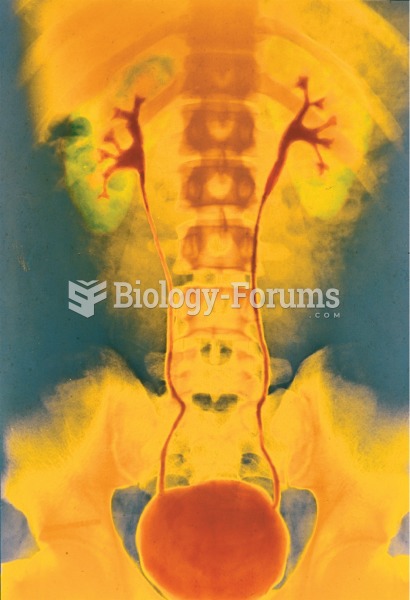 Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra