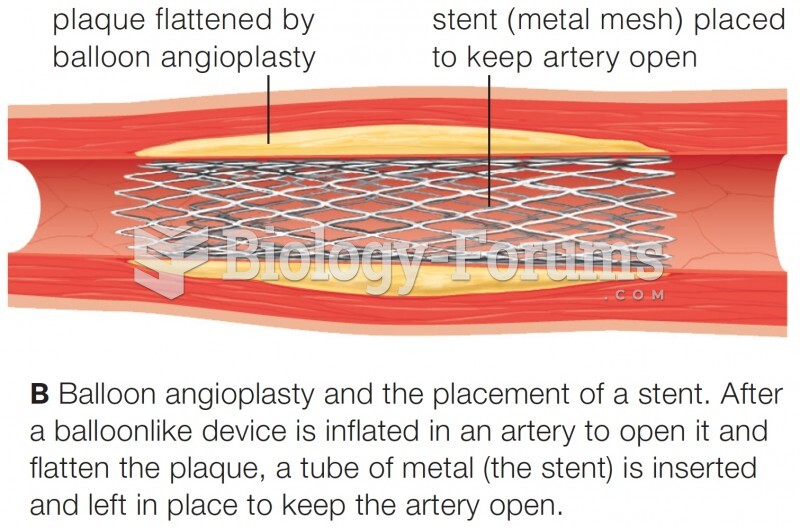
|
|
|
The familiar sounds of your heart are made by the heart's valves as they open and close.
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
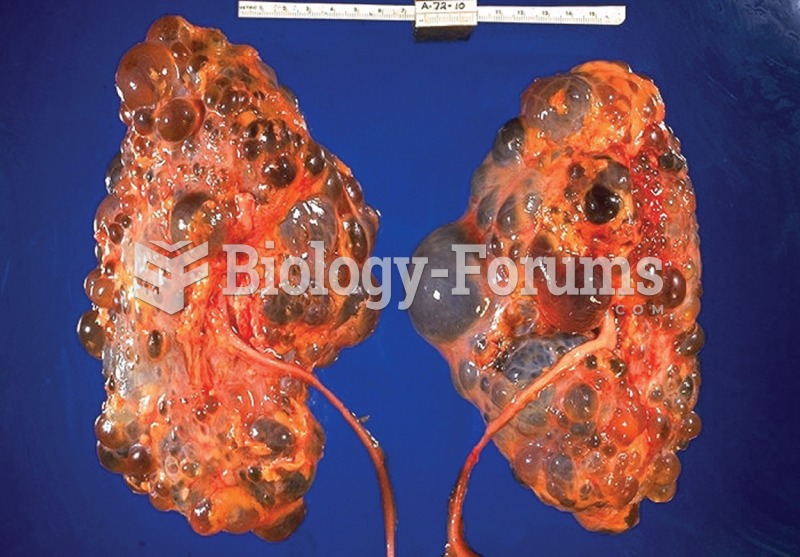 Polycystic kidney disease. Notice the presence of numerous fluid-filled sacs, or cysts, in these kid
Polycystic kidney disease. Notice the presence of numerous fluid-filled sacs, or cysts, in these kid
 A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
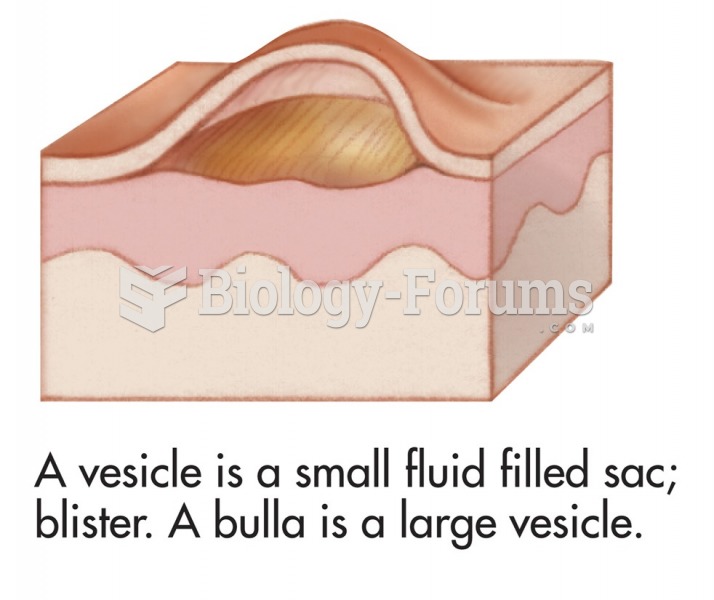 Common skin signs are often evidence of an illness or disorder. A vesicle is a small fluid filled ...
Common skin signs are often evidence of an illness or disorder. A vesicle is a small fluid filled ...
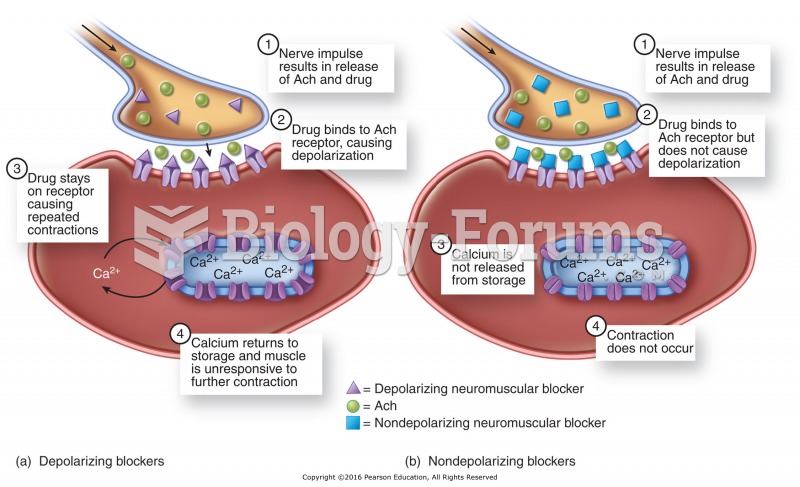 Mechanism of action of neuromuscular blockers: (a) Depolarizing neuromuscular blocker occupies Ach ...
Mechanism of action of neuromuscular blockers: (a) Depolarizing neuromuscular blocker occupies Ach ...