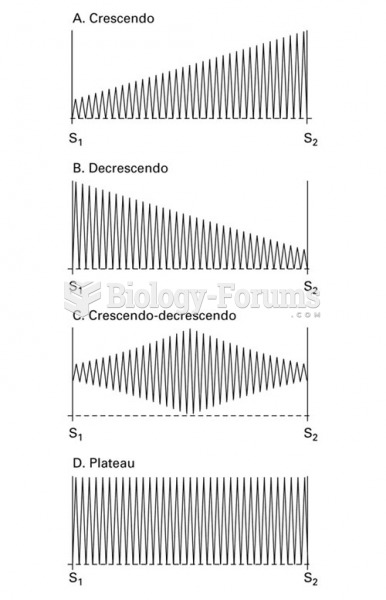
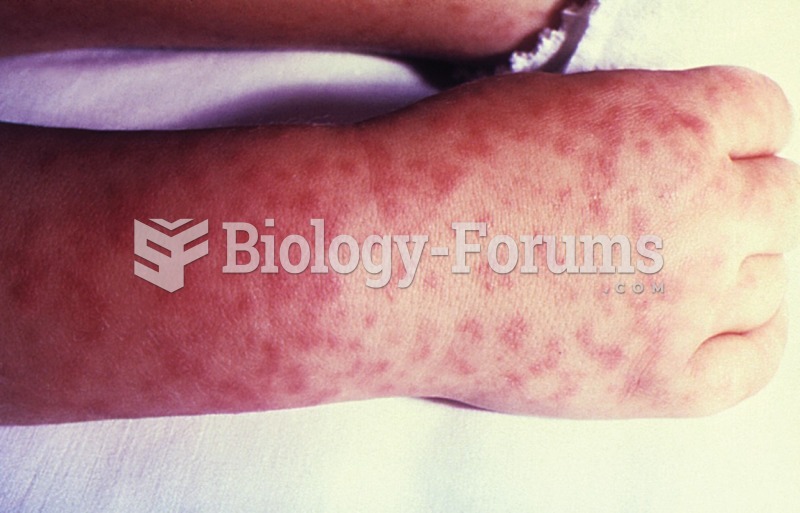
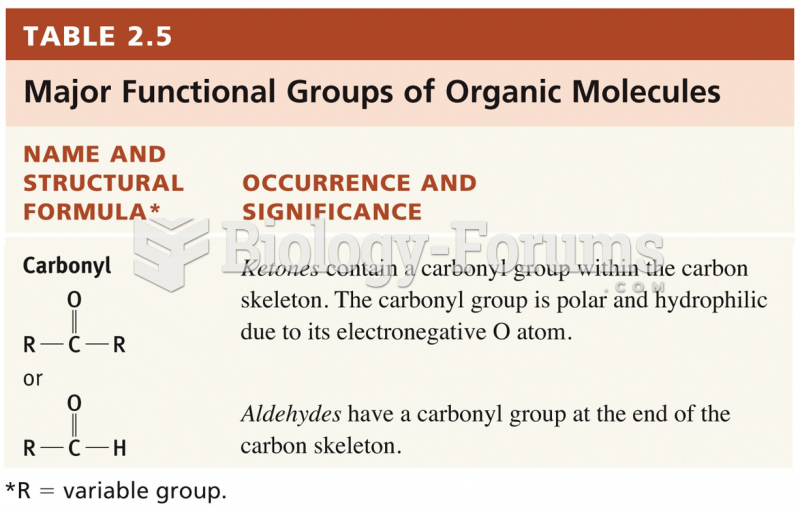
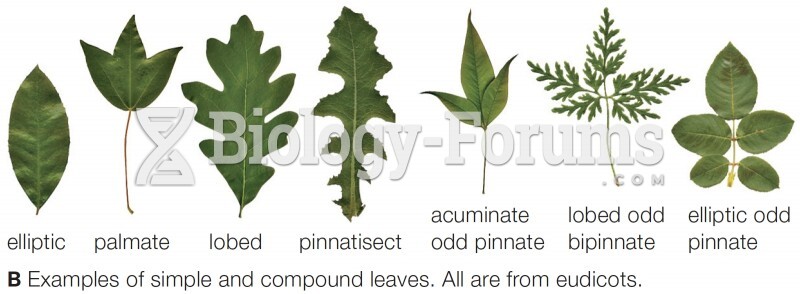
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.