This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
Did you know?
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
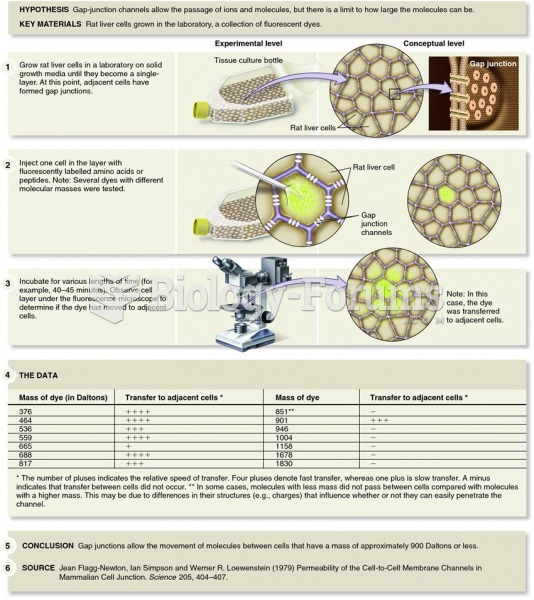 Use of fluorescent molecules by Lowenstein and colleagues to determine the size of gap-junction chan
Use of fluorescent molecules by Lowenstein and colleagues to determine the size of gap-junction chan
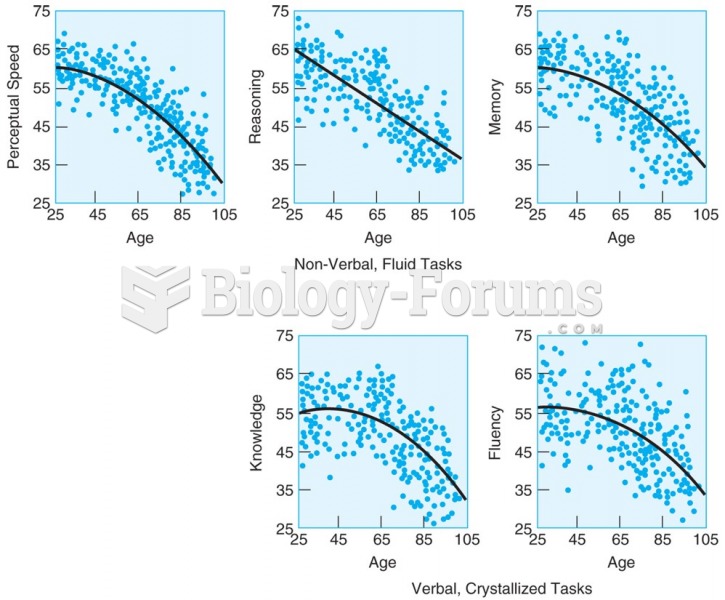 Adults in the Berlin Study of Aging show earlier declines on tests of nonverbal, fluid tasks (upper ...
Adults in the Berlin Study of Aging show earlier declines on tests of nonverbal, fluid tasks (upper ...