|
|
|
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Chronic necrotizing aspergillosis has a slowly progressive process that, unlike invasive aspergillosis, does not spread to other organ systems or the blood vessels. It most often affects middle-aged and elderly individuals, spreading to surrounding tissue in the lungs. The disease often does not respond to conventionally successful treatments, and requires individualized therapies in order to keep it from becoming life-threatening.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
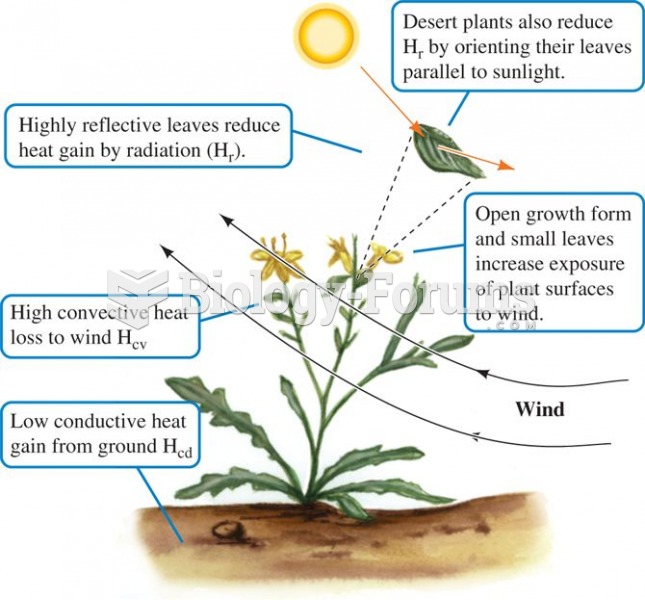 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
 The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf