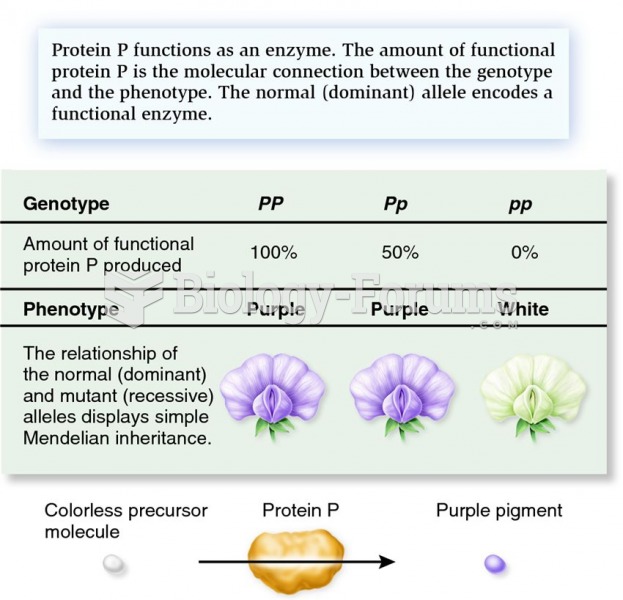
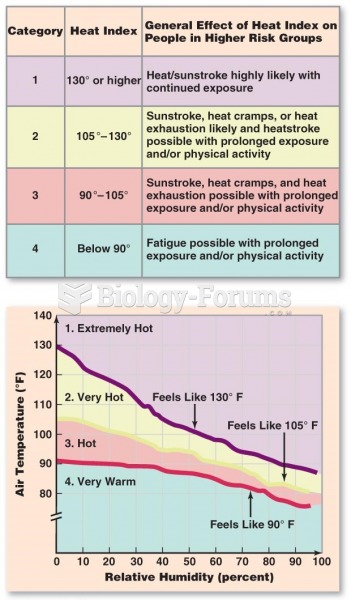
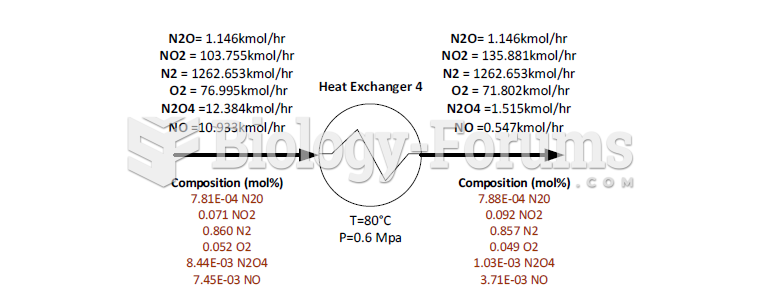
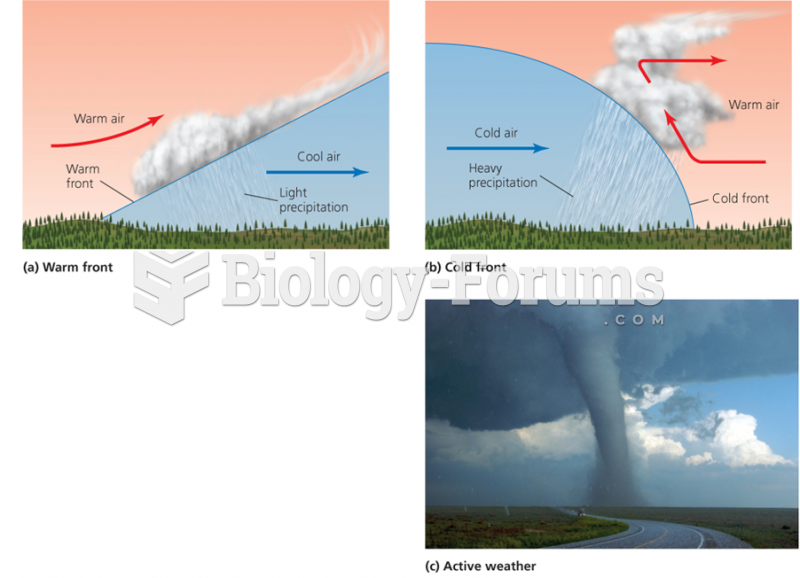
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.