|
|
|
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
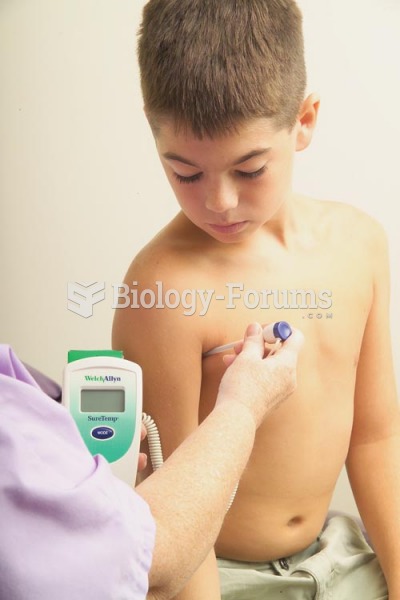
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
The Food and Drug Administration has approved Risperdal, an adult antipsychotic drug, for the symptomatic treatment of irritability in children and adolescents with autism. The approval is the first for the use of a drug to treat behaviors associated with autism in children. These behaviors are included under the general heading of irritability and include aggression, deliberate self-injury, and temper tantrums.