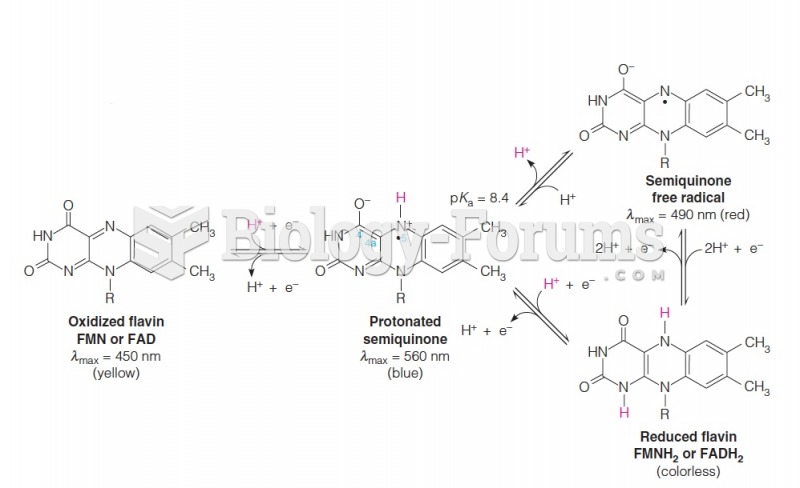
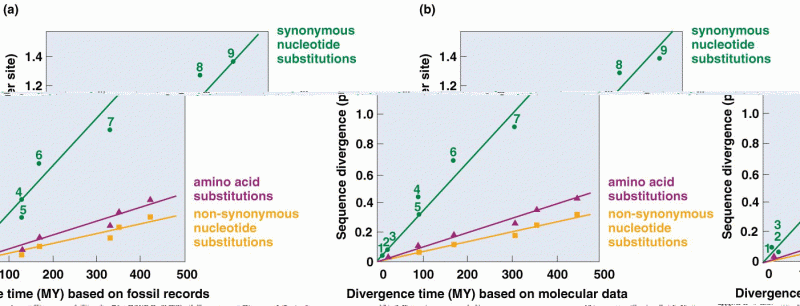
|
|
|
Anesthesia awareness is a potentially disturbing adverse effect wherein patients who have been paralyzed with muscle relaxants may awaken. They may be aware of their surroundings but unable to communicate or move. Neurologic monitoring equipment that helps to more closely check the patient's anesthesia stages is now available to avoid the occurrence of anesthesia awareness.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.