|
|
|
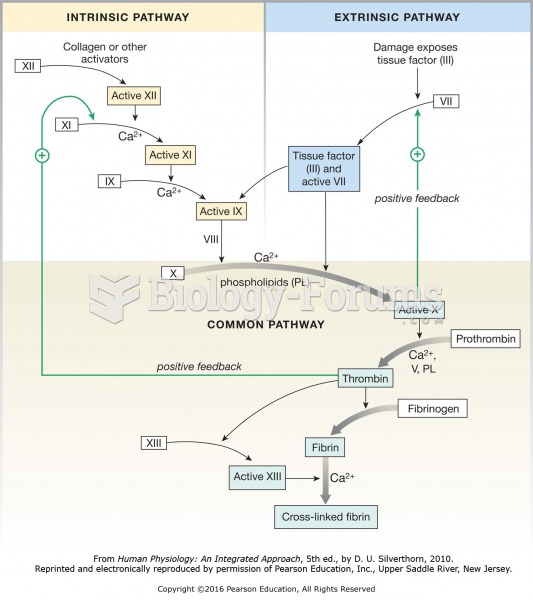
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Your heart beats over 36 million times a year.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.
There can actually be a 25-hour time difference between certain locations in the world. The International Date Line passes between the islands of Samoa and American Samoa. It is not a straight line, but "zig-zags" around various island chains. Therefore, Samoa and nearby islands have one date, while American Samoa and nearby islands are one day behind. Daylight saving time is used in some islands, but not in others—further shifting the hours out of sync with natural time.
 Approximately 1 pound (0.45 kg) of ground elk meat formed into patties; note the relatively small fa
Approximately 1 pound (0.45 kg) of ground elk meat formed into patties; note the relatively small fa
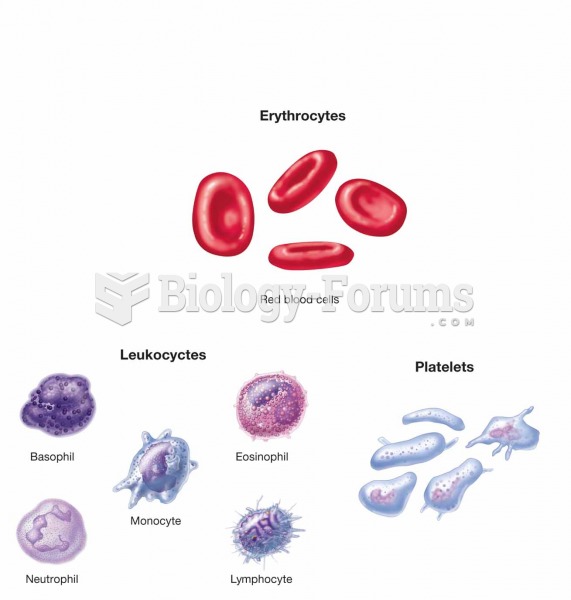 Formed elements of blood: erythrocytes, leukocytes (neutrophils, eosinophils, basophils, lymphocytes
Formed elements of blood: erythrocytes, leukocytes (neutrophils, eosinophils, basophils, lymphocytes