|
|
|
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
Your skin wrinkles if you stay in the bathtub a long time because the outermost layer of skin (which consists of dead keratin) swells when it absorbs water. It is tightly attached to the skin below it, so it compensates for the increased area by wrinkling. This happens to the hands and feet because they have the thickest layer of dead keratin cells.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
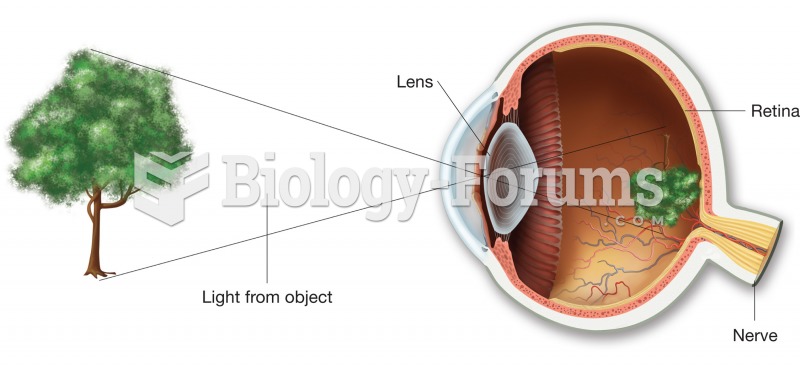 The image formed on the retina is inverted. The brain rights the image as part of the interpretation
The image formed on the retina is inverted. The brain rights the image as part of the interpretation
 The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p
The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p