|
|
|
Did you know?
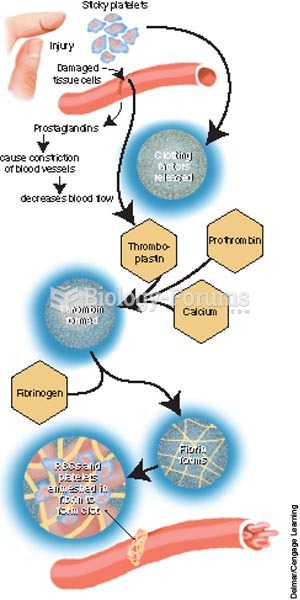
There are 60,000 miles of blood vessels in every adult human.
Did you know?
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Did you know?
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
The FDA recognizes 118 routes of administration.