|
|
|
Though newer “smart” infusion pumps are increasingly becoming more sophisticated, they cannot prevent all programming and administration errors. Health care professionals that use smart infusion pumps must still practice the rights of medication administration and have other professionals double-check all high-risk infusions.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
People about to have surgery must tell their health care providers about all supplements they take.
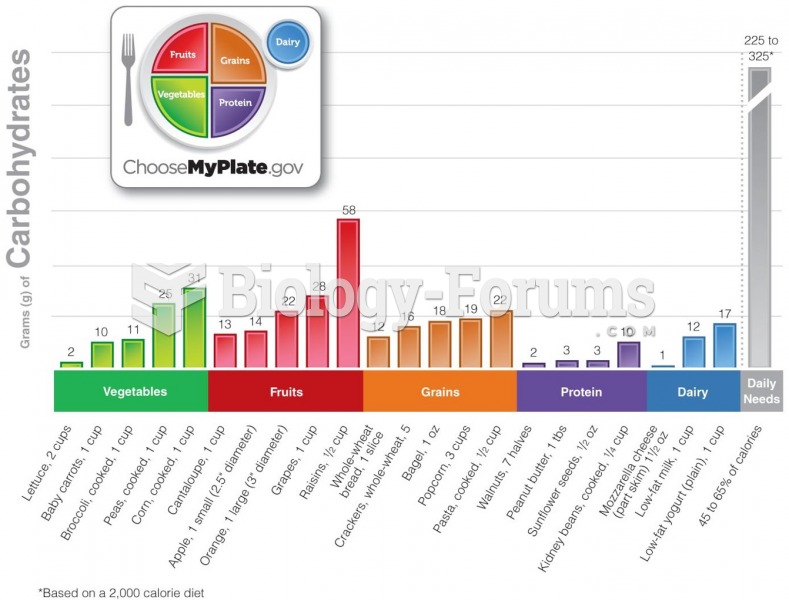
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
Women are 50% to 75% more likely than men to experience an adverse drug reaction.