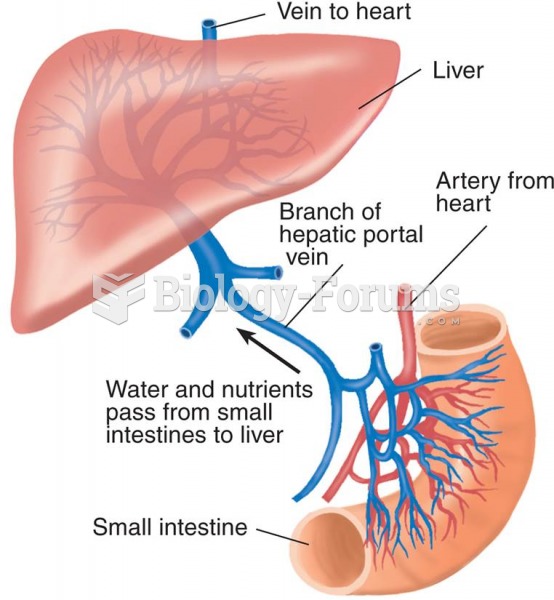
|
|
|
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
 Water striders serve as a model organism for the study of sexual conflict between males and females.
Water striders serve as a model organism for the study of sexual conflict between males and females.
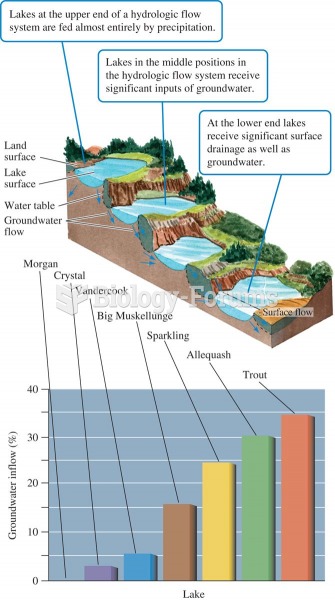 Lake position in the landscape and proportion of water received as groundwater (data from Webster et
Lake position in the landscape and proportion of water received as groundwater (data from Webster et