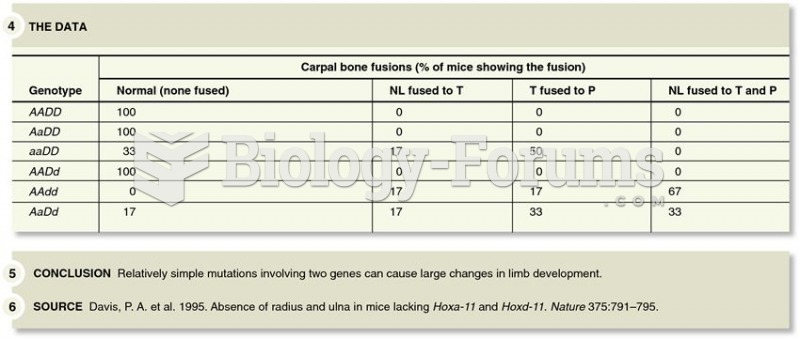
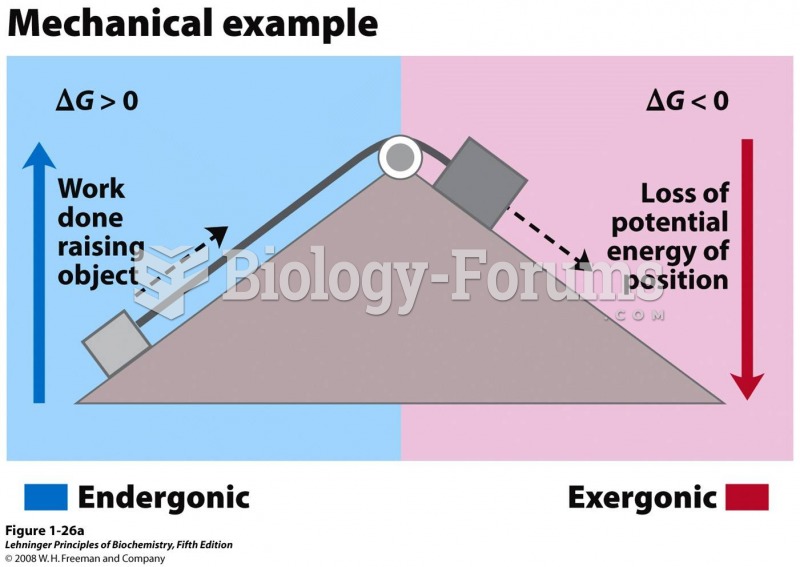
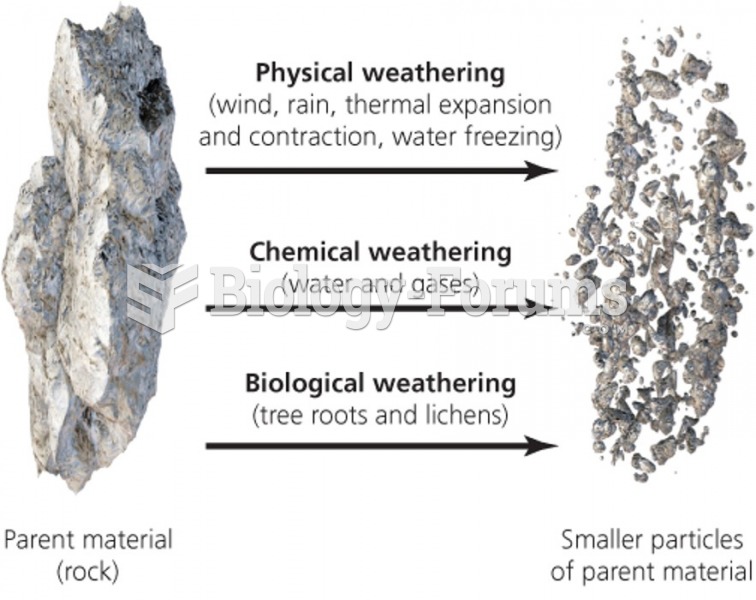
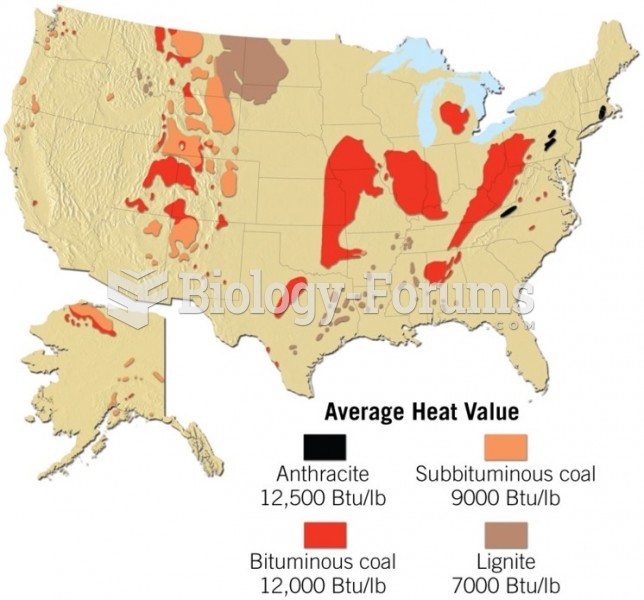
|
|
|
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
Complications of influenza include: bacterial pneumonia, ear and sinus infections, dehydration, and worsening of chronic conditions such as asthma, congestive heart failure, or diabetes.
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.