This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
Did you know?
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
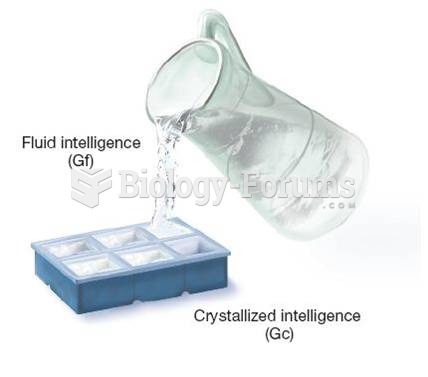
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.