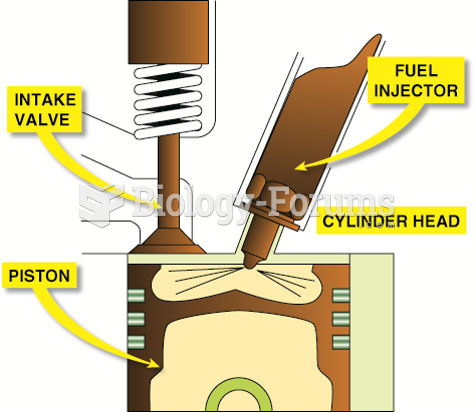
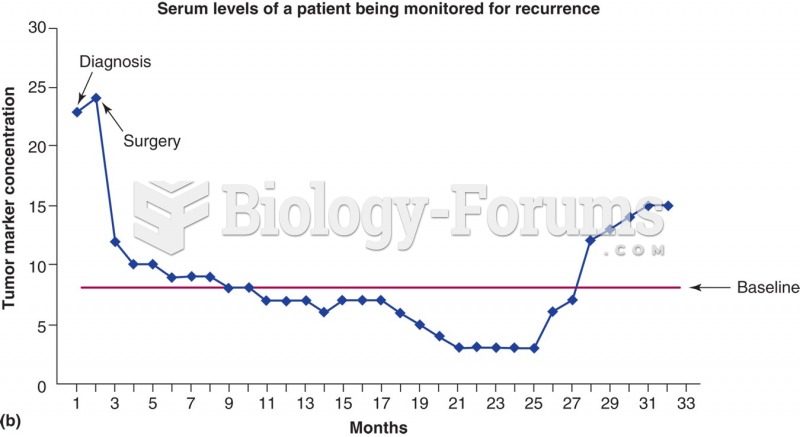
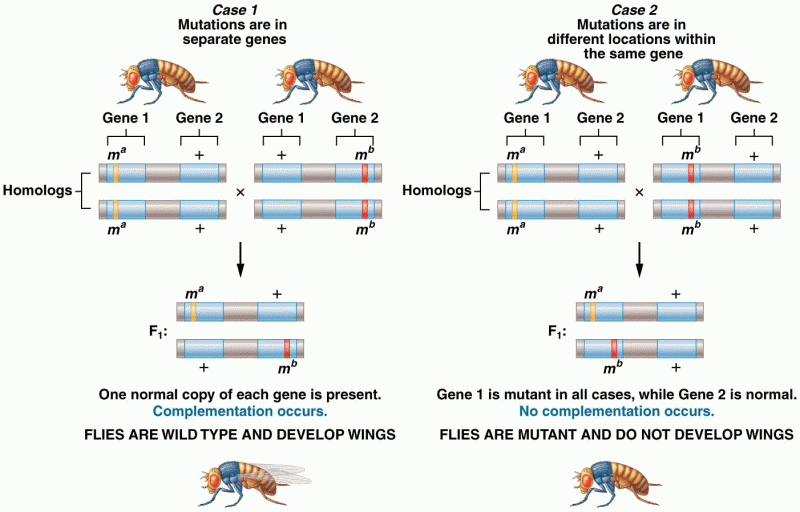
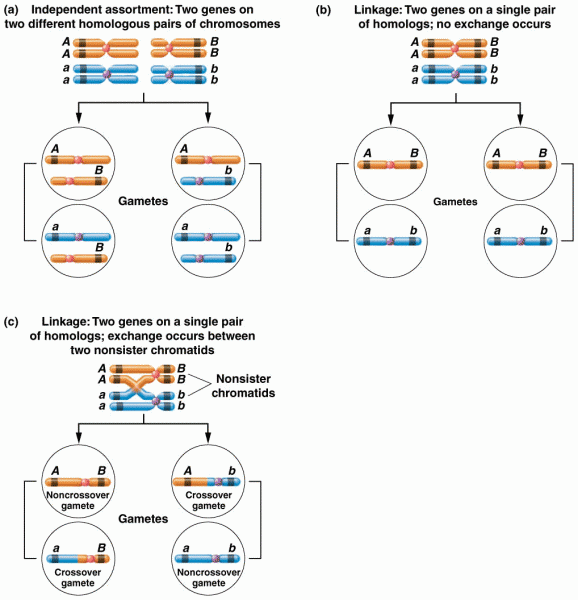
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.