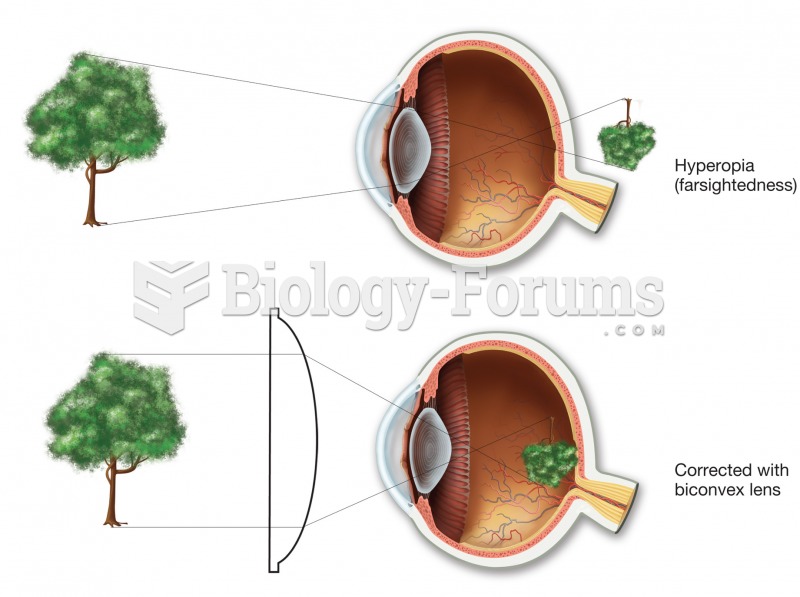
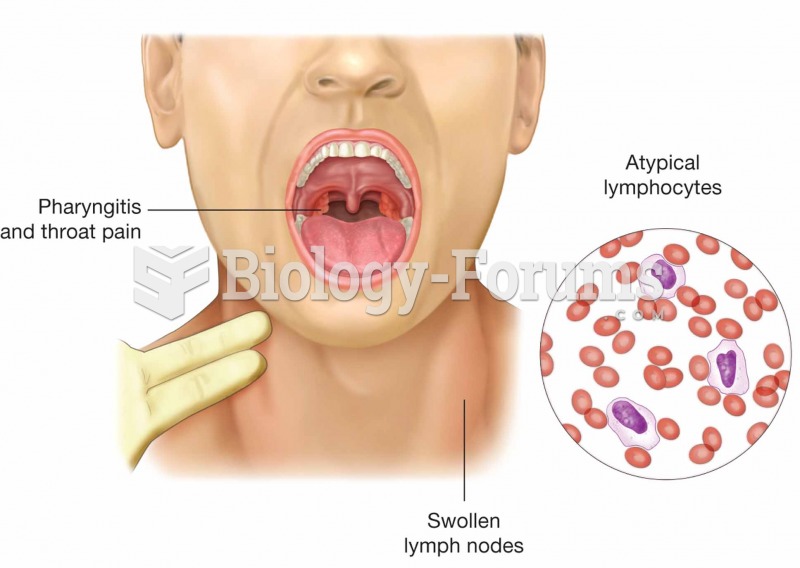
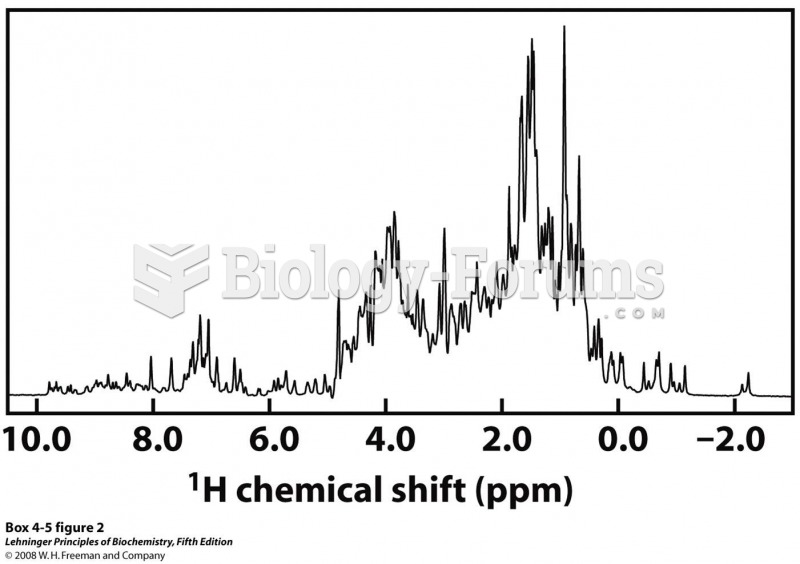
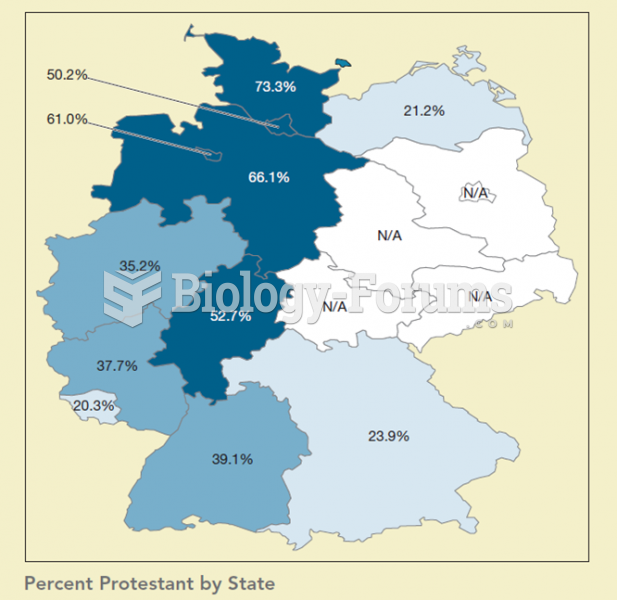
|
|
|
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").