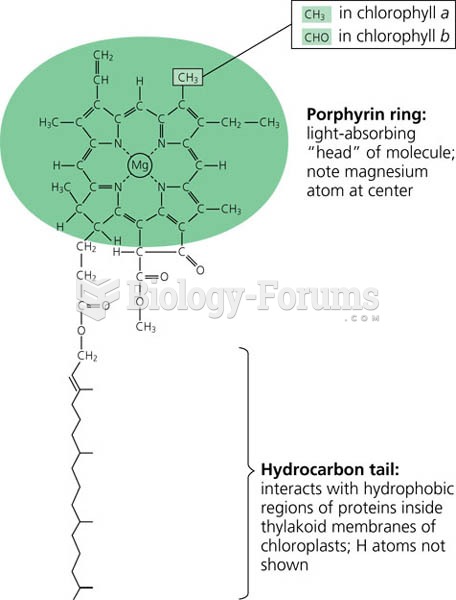
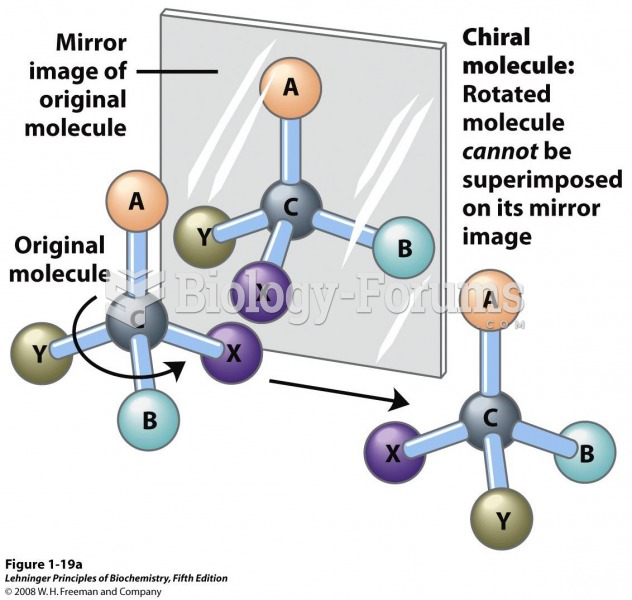
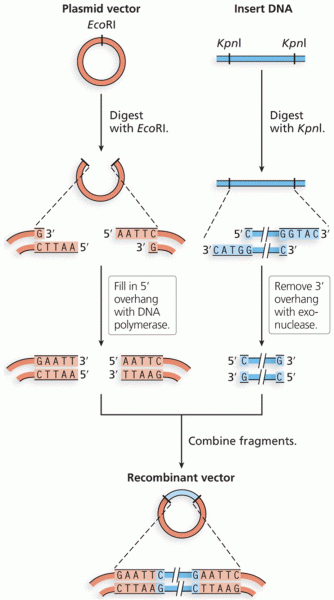
|
|
|
There are more sensory neurons in the tongue than in any other part of the body.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Before a vaccine is licensed in the USA, the Food and Drug Administration (FDA) reviews it for safety and effectiveness. The CDC then reviews all studies again, as well as the American Academy of Pediatrics and the American Academy of Family Physicians. Every lot of vaccine is tested before administration to the public, and the FDA regularly inspects vaccine manufacturers' facilities.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.