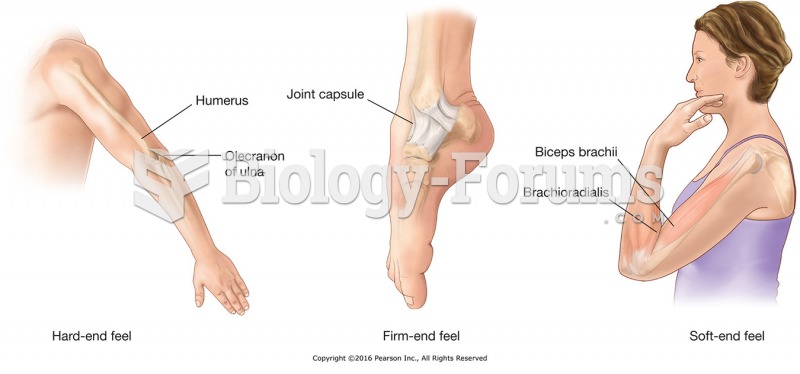
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
Did you know?
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.