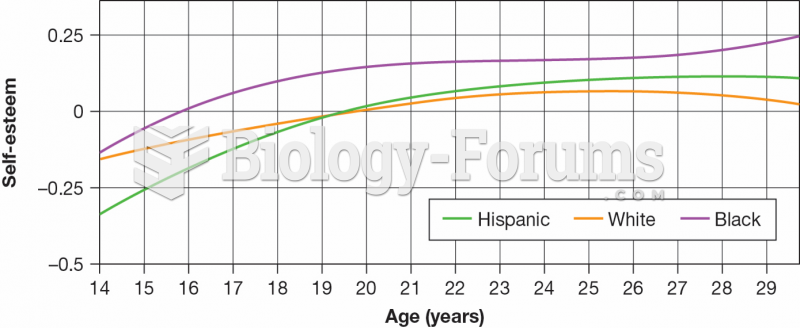
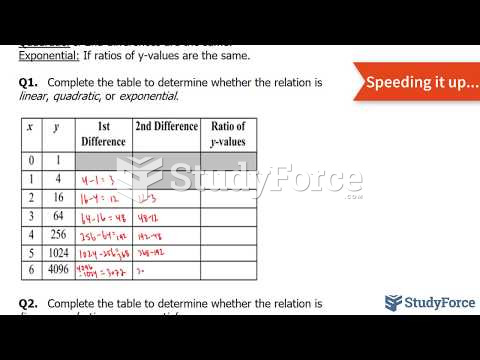
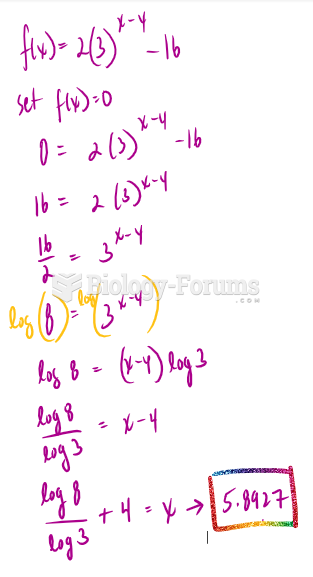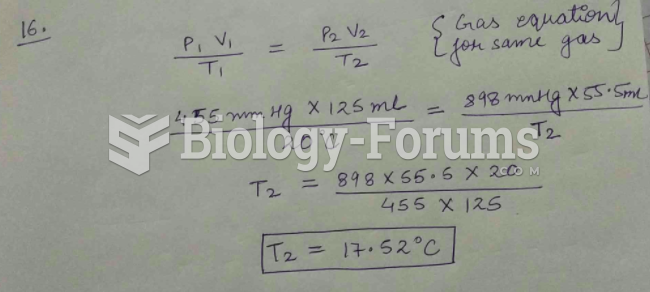This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Did you know?
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Did you know?
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.