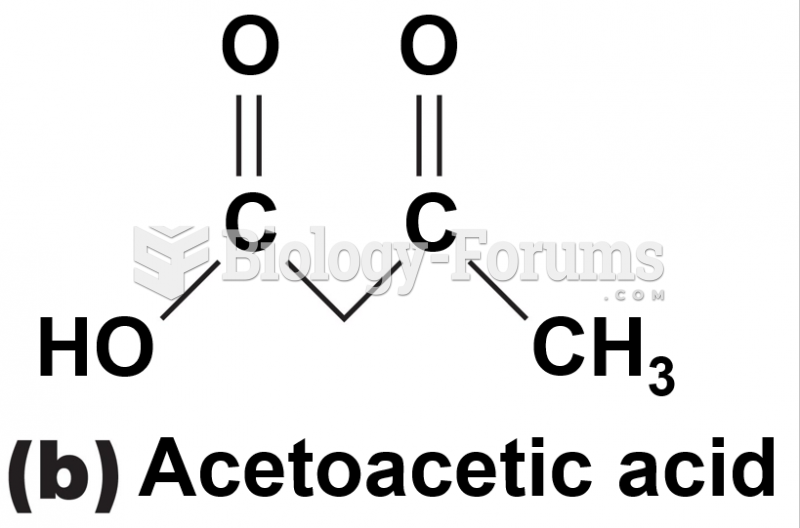
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.