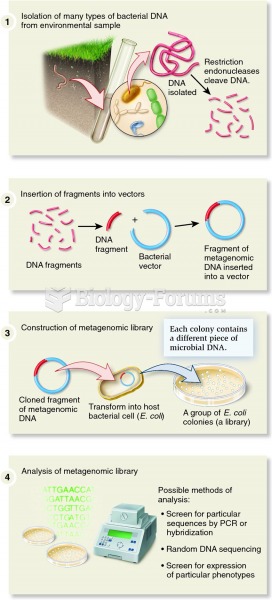
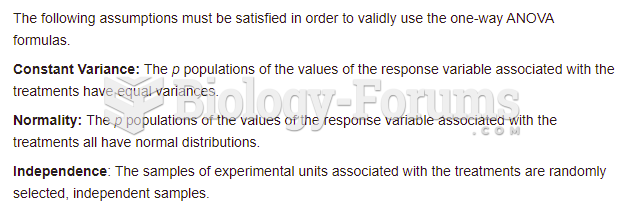
|
|
|
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.