|
|
|
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
A cataract is a clouding of the eyes' natural lens. As we age, some clouding of the lens may occur. The first sign of a cataract is usually blurry vision. Although glasses and other visual aids may at first help a person with cataracts, surgery may become inevitable. Cataract surgery is very successful in restoring vision, and it is the most frequently performed surgery in the United States.
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
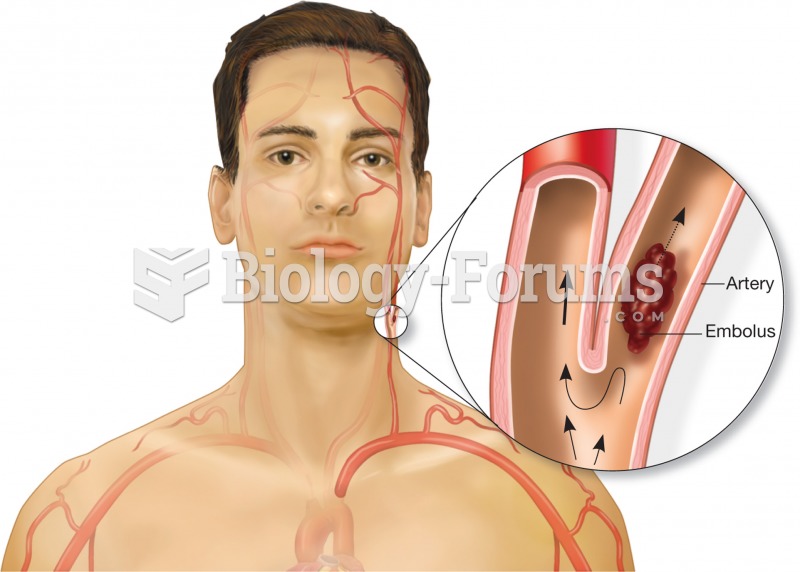 Illustration of an embolus floating in an artery. The embolus will become lodged in a blood vessel t
Illustration of an embolus floating in an artery. The embolus will become lodged in a blood vessel t
 Rule of Nines. A method for determining percentage of body burned. Each different colored section re
Rule of Nines. A method for determining percentage of body burned. Each different colored section re