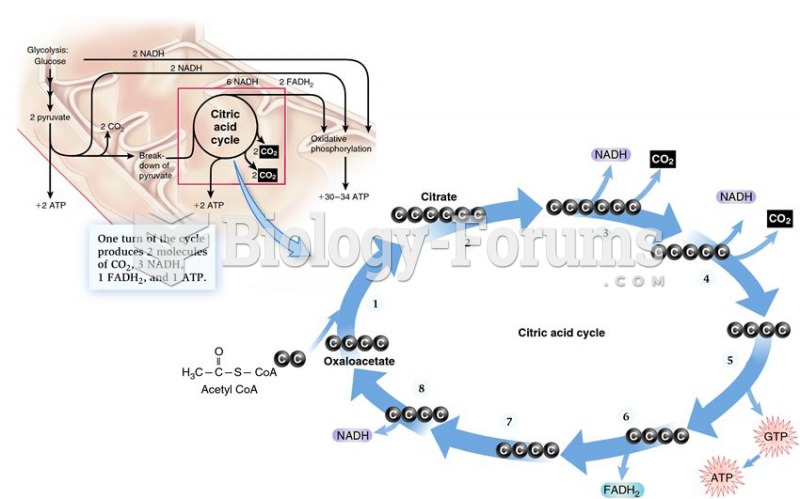
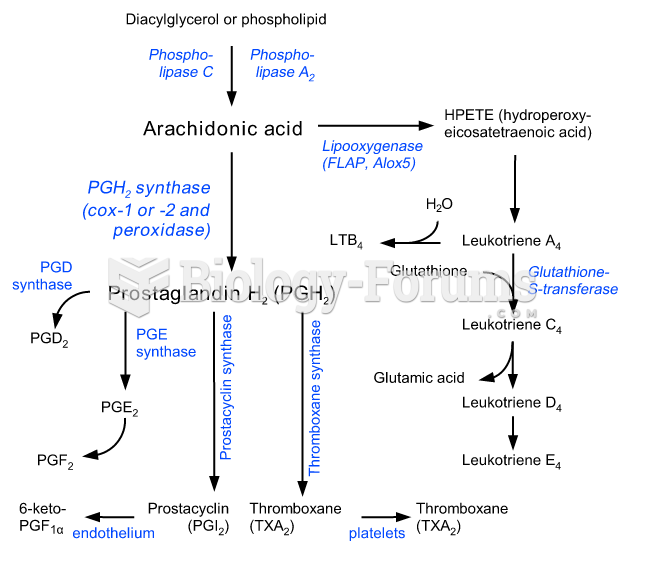
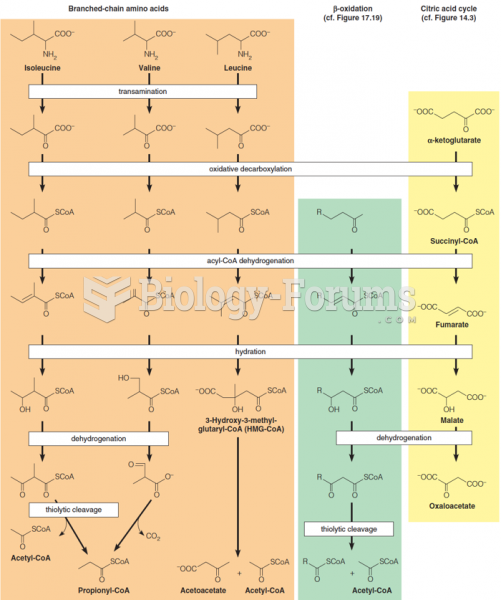
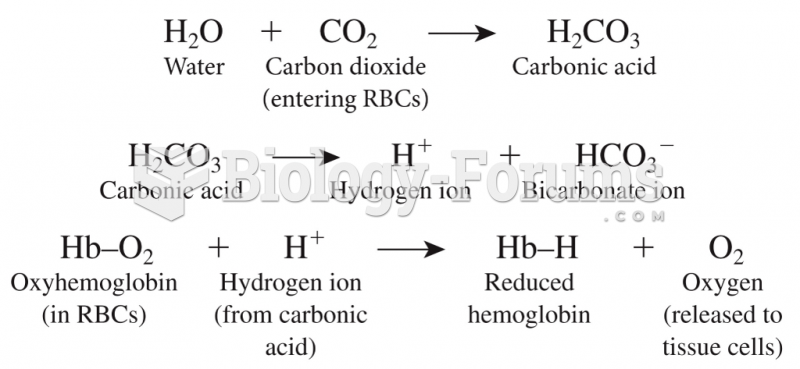
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
Malaria mortality rates are falling. Increased malaria prevention and control measures have greatly improved these rates. Since 2000, malaria mortality rates have fallen globally by 60% among all age groups, and by 65% among children under age 5.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.