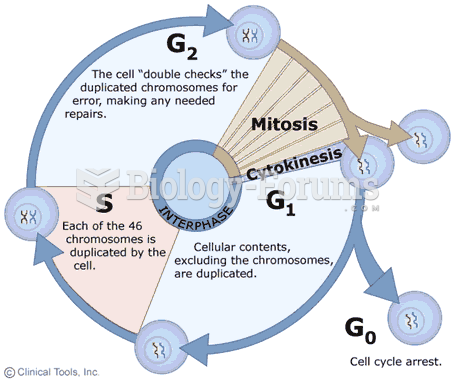
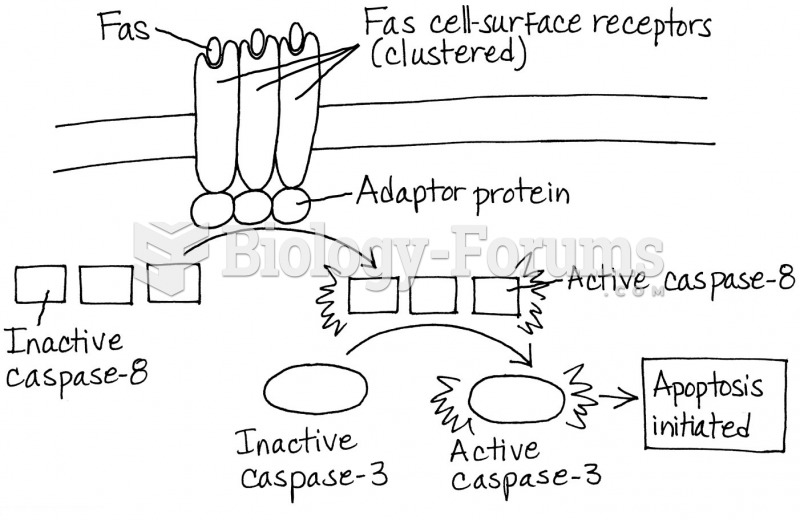
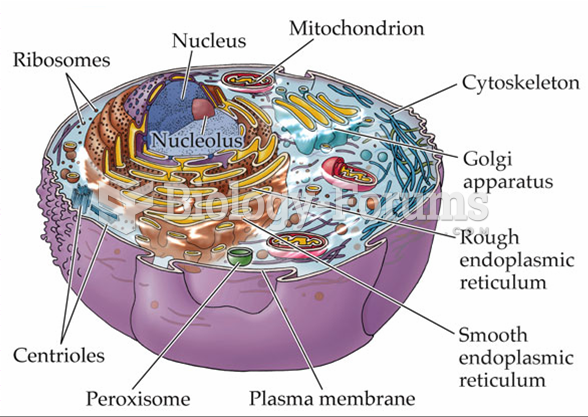
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.