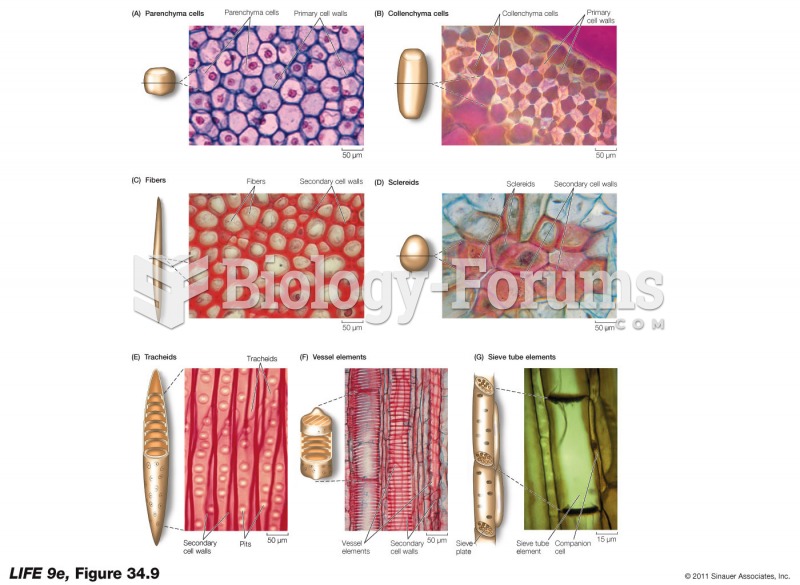
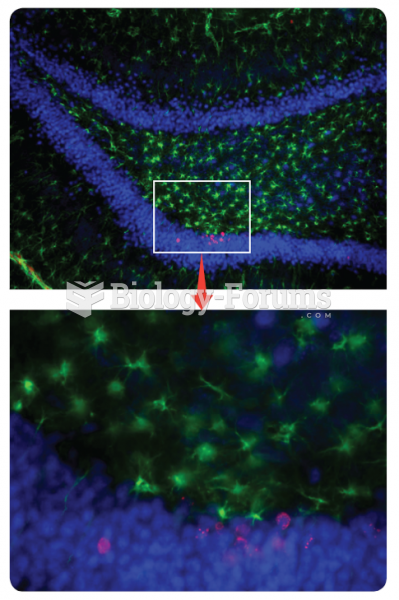
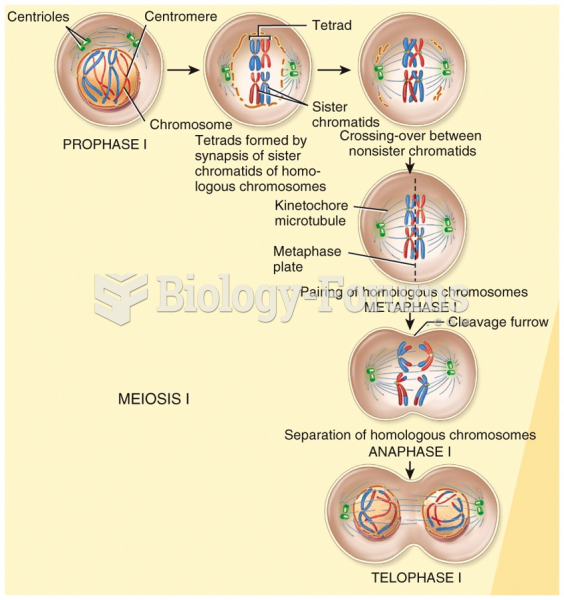
|
|
|
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Green tea is able to stop the scent of garlic or onion from causing bad breath.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
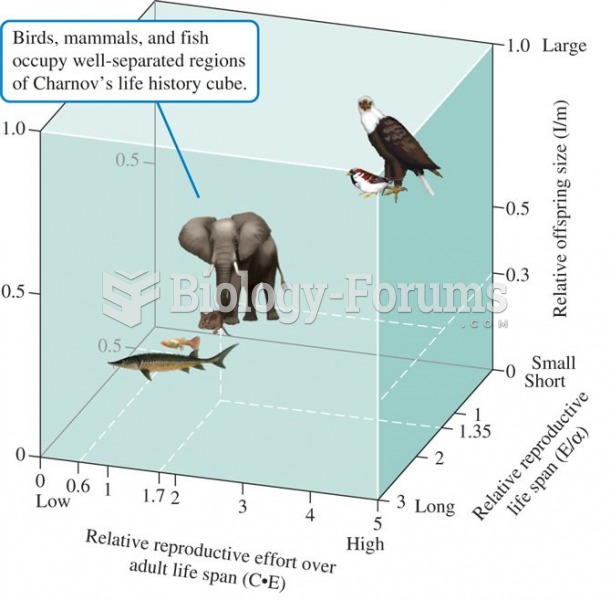 Life-history cube, a classification of fish, mammals, and altricial birds based on three dimensionle
Life-history cube, a classification of fish, mammals, and altricial birds based on three dimensionle
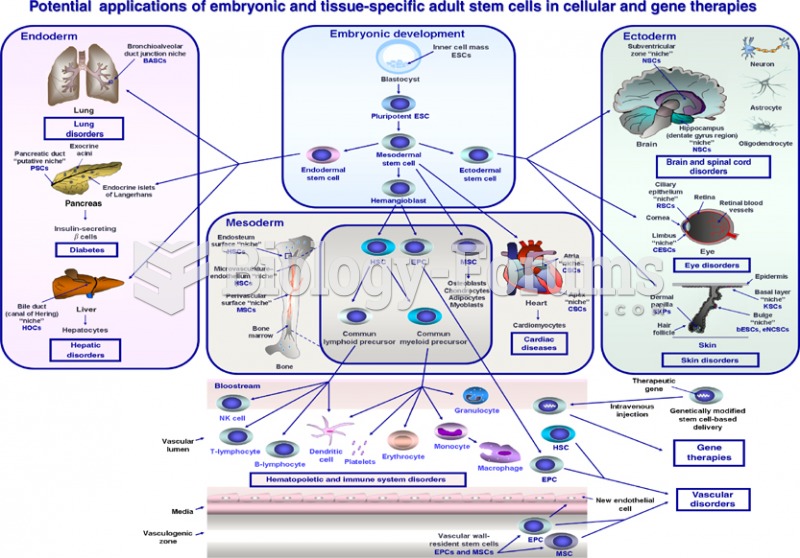 Potential applications of embryonic and tissue specific adult stem cell in cellular and gene theraph
Potential applications of embryonic and tissue specific adult stem cell in cellular and gene theraph