This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
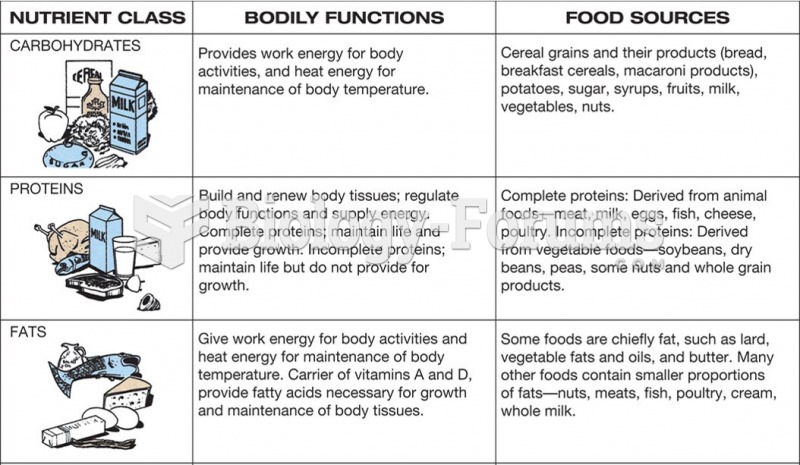
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
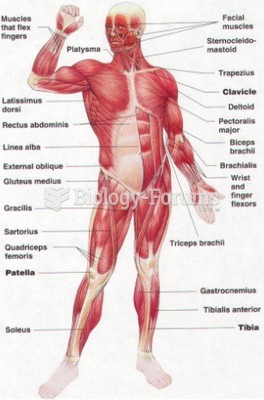
The average adult has about 21 square feet of skin.
Did you know?
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
Did you know?
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.