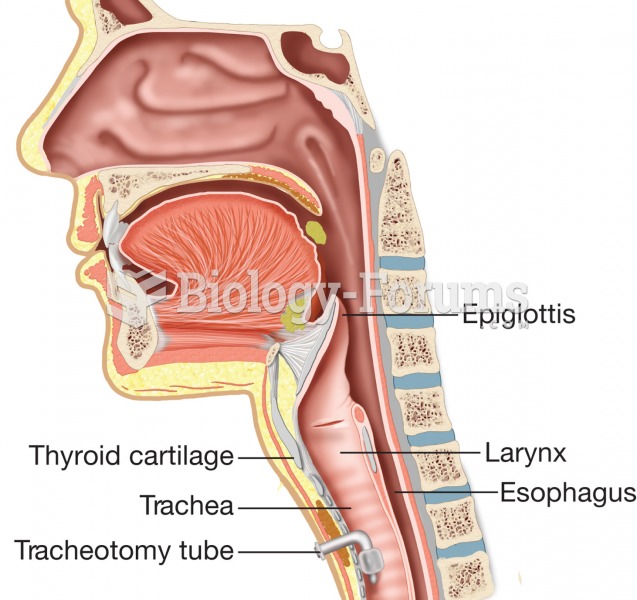
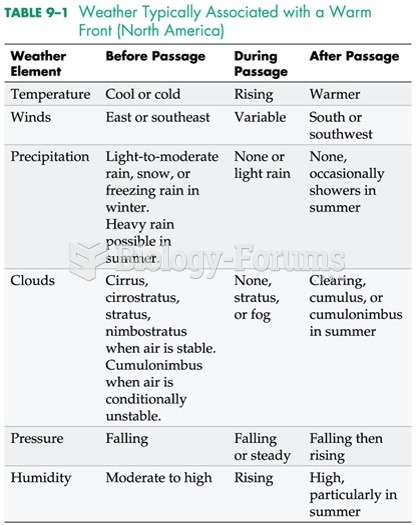
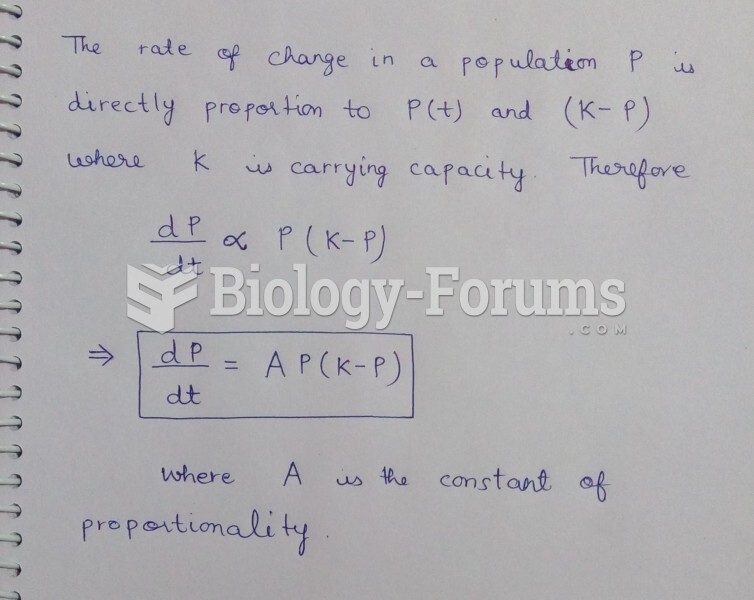
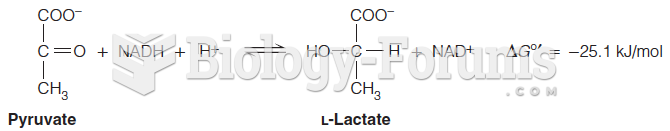This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Did you know?
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Did you know?
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.