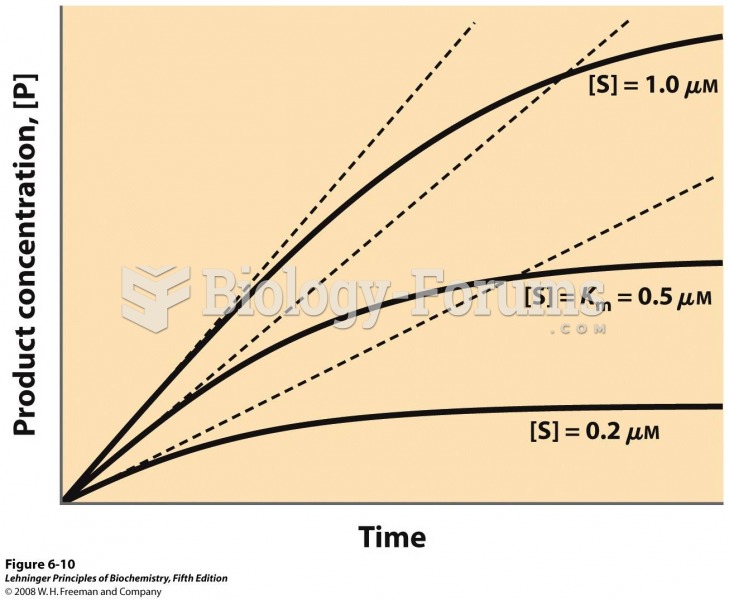
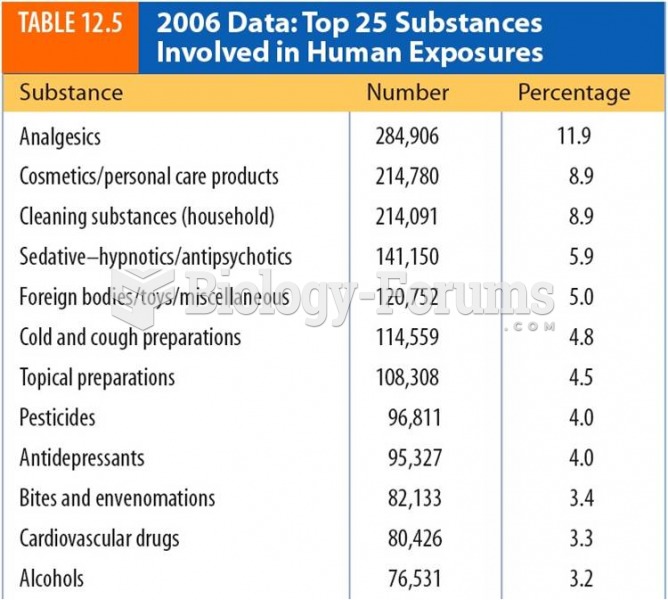
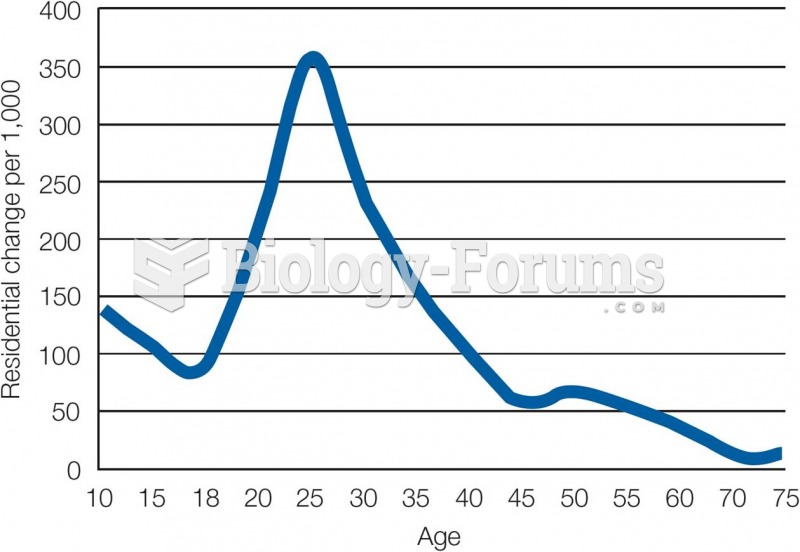
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.