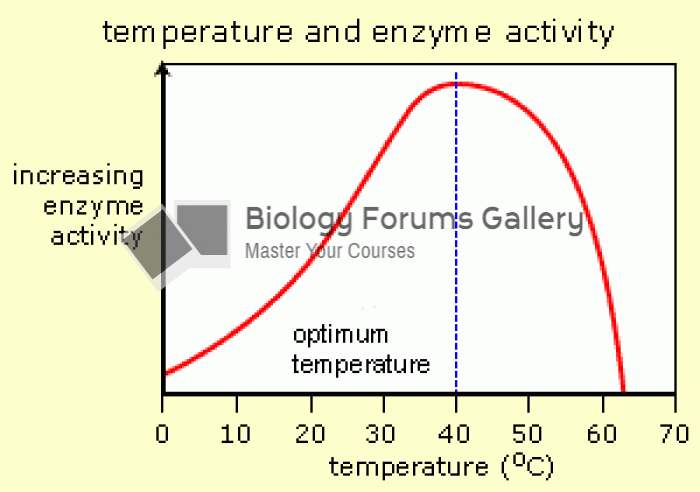
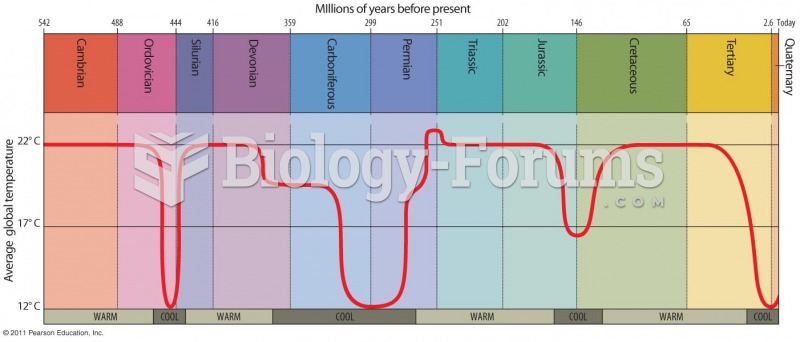
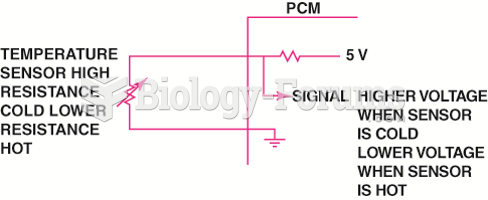
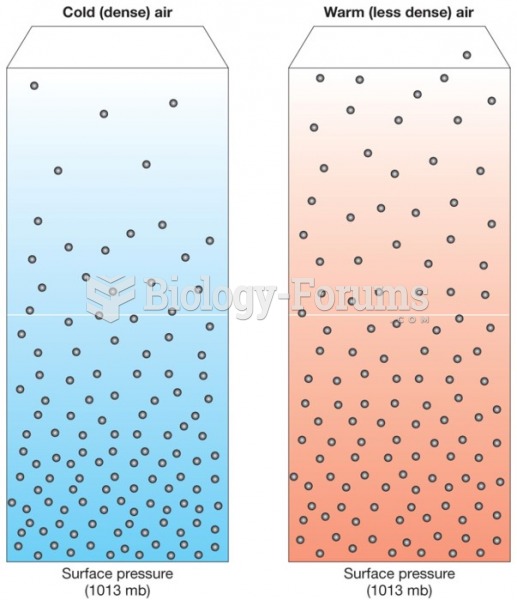
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.