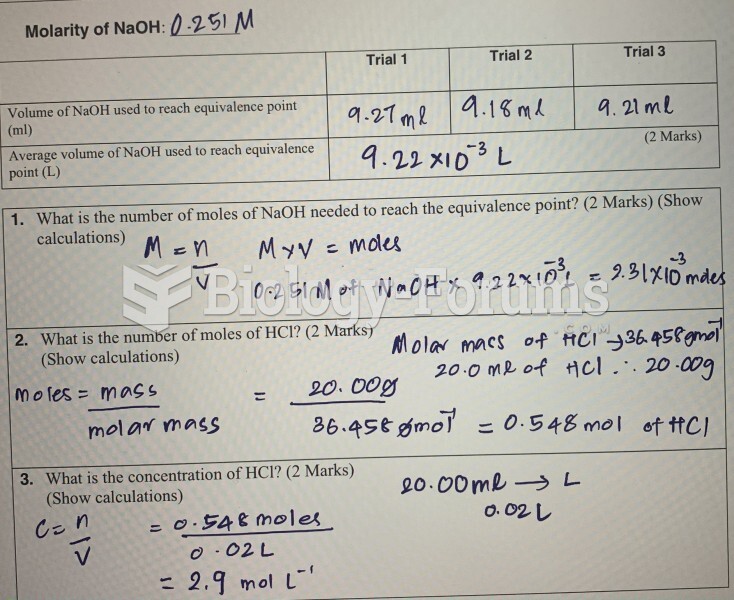
|
|
|
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
In 1864, the first barbiturate (barbituric acid) was synthesized.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Cyanide works by making the human body unable to use oxygen.