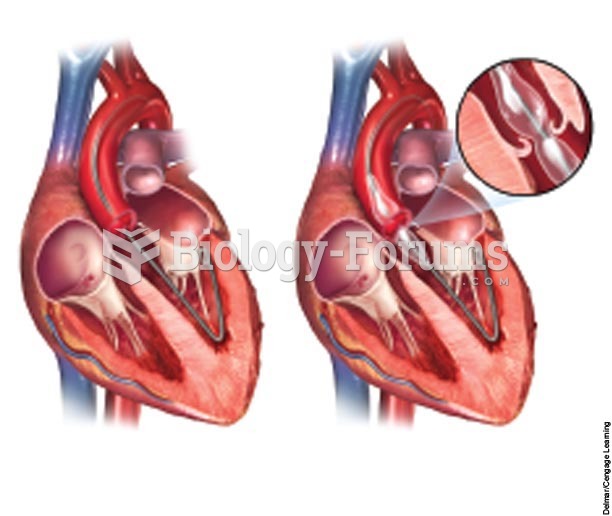
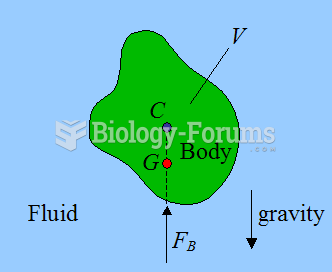
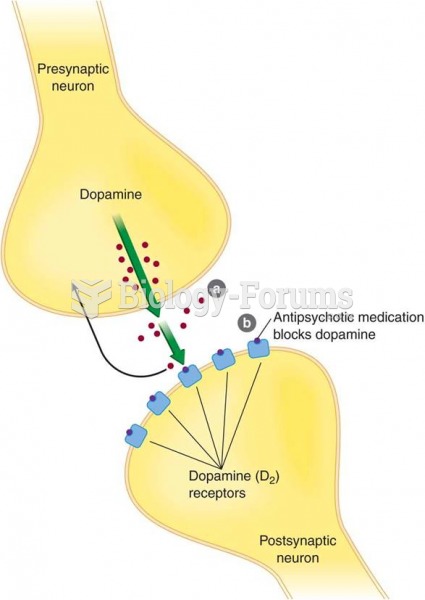
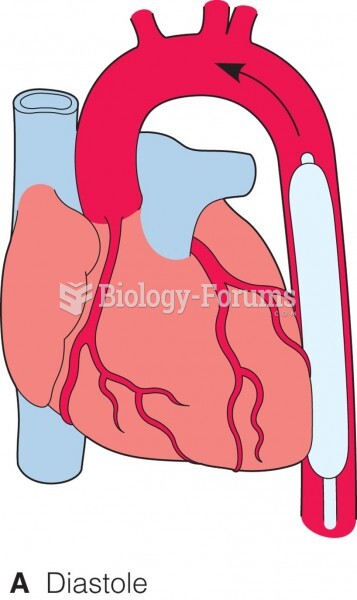
|
|
|
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Pink eye is a term that refers to conjunctivitis, which is inflammation of the thin, clear membrane (conjunctiva) over the white part of the eye (sclera). It may be triggered by a virus, bacteria, or foreign body in the eye. Antibiotic eye drops alleviate bacterial conjunctivitis, and antihistamine allergy pills or eye drops help control allergic conjunctivitis symptoms.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.