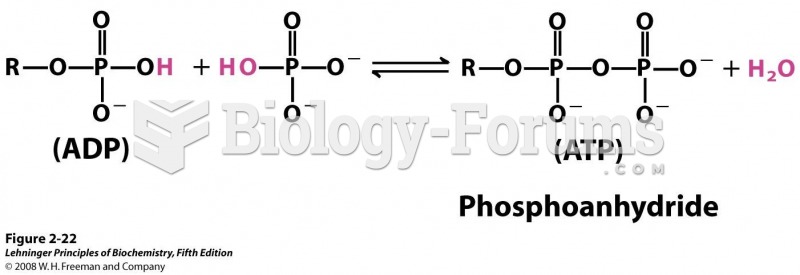
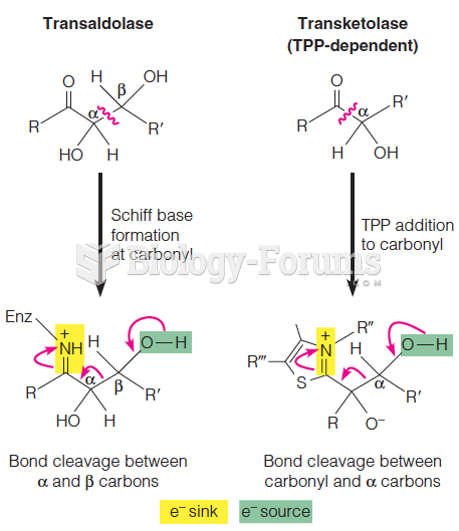
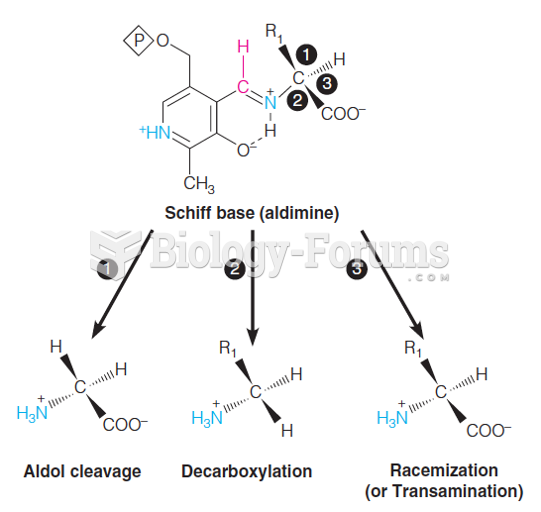
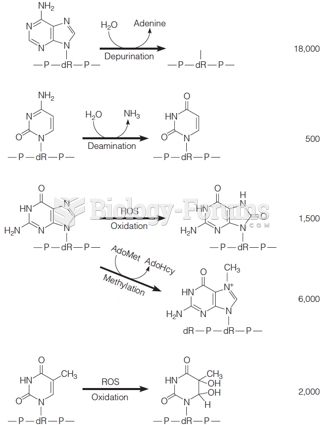
|
|
|
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.